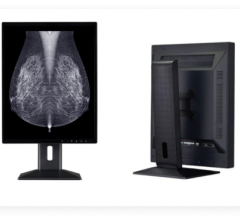
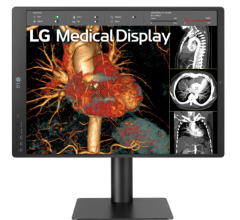
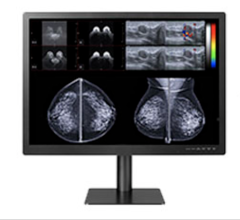
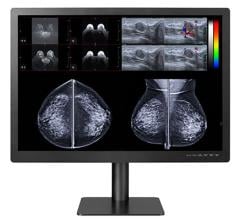
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to 21-inch MultiSync MD211C3, a medical-grade widescreen diagnostic display monitor built for diagnostic radiology applications.
This 3-megapixel, grayscale LED backlit MultiSync MD Series model is factory calibrated to the digital imaging and communications in medicine (DICOM) grayscale function for luminance and includes a new, smaller front sensor to maintain a calibrated brightness, a one button Quick Quality Assurance (QA) Test for easy DICOM evaluation and free GammaCompMD QA software.
The SA-SFT IPS panel offers 2048-by-1,536 resolution. The DVI-D and DisplayPort connectors for video and two-port USB hub enable connectivity for any type of workstation. The MD211G3’s built-in front sensor constantly monitors and maintains brightness for optimal DICOM GSDF calibration, while its four-way ergonomic stand allows users to adjust for comfort and preference accordingly.
MultiSync MD211C3 received clearance in September 2013.
For more information: www.necdisplay.com

 March 12, 2024
March 12, 2024