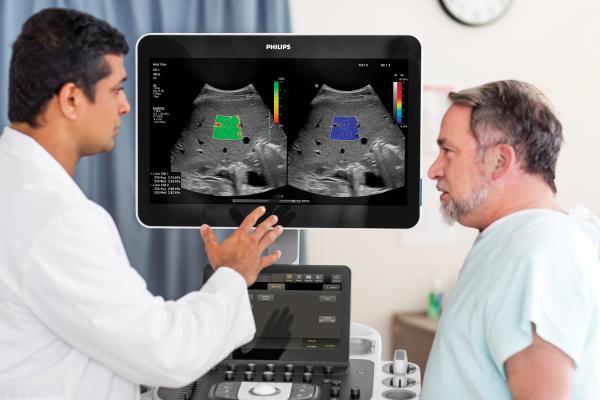
November 18, 2021 — Royal Philips, a leader in health technology, announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Liver Fat Quantification tools as part of the latest release of its ultrasound systems EPIQ Elite and Affiniti, bringing the cost and accessibility advantages of sonography to the diagnosis of early-stage liver disease. Featured at this year’s Radiological Society of North America (RSNA) annual meeting, the new tools will allow clinicians to track liver health.
Fatty liver disease is the most common and earliest stage of chronic liver disease. The incidence of non-alcoholic fatty liver disease (NAFLD) is increased by various risk factors, including Type 2 diabetes and obesity. It is estimated that NAFLD may be present in about 25% of the global population.1 NAFLD includes milder fatty liver disease, as well as more severe forms that include inflammation, a condition called Non-Alcoholic Steatohepatitis (NASH), and fibrosis. Research suggests that early intervention enables patients to adopt lifestyle changes that can help to prevent liver disease,1 while early assessment of fatty liver is key to potentially reversing this progression of liver disease.2
"With a quantitative way of measuring liver fat, it is a lot easier for us to let the referring physician know where the patient is quantitatively on the NAFLD spectrum,” said Richard G. Barr, M.D., Ph.D., president, Radiology Consultant Inc. and medical director at Southwoods Imaging. “With traditional gray scale imaging, we could only tell if the liver had a high degree of fatty infiltration or if it was normal, but it was very hard to assess whether fatty liver disease was mild, moderate or severe. Attenuation imaging now gives us a numerical value that will enable us to follow the patient over time. With the combination of quantitative fat assessment and liver stiffness the probability of NASH can be assessed.”
With the extended remote functionality of Collaboration Live on both the EPIQ and Affiniti platforms, technicians can also securely access on-demand, real-time guidance and decision support to enhance diagnostic confidence and workflow efficiency during exams.
“[The] announcement demonstrates the continued advancement of our ultrasound portfolio to increase diagnostic confidence and workflow efficiency,” said Jeff Cohen, general manager of ultrasound at Philips. “Accessible ultrasound-based Liver Fat Quantification is a screening and early diagnostic tool that will allow many more patients to take their health into their own hands by making simple lifestyle changes.”
Enhancing the Diagnosis and Treatment of Early-stage and Advanced Liver Disease
Philips Ultrasound Systems EPIQ and Affiniti on Release 9.0 now support radiologists and hepatologists in the diagnosis and treatment from early-stage to advanced liver disease, making it easier to perform longitudinal studies to assess liver disease progression. With this latest release, the company further strengthens its liver solution and expands its full availability to the Affiniti 70 and Affiniti 50—supported by the innovative PureWave C5-1 Curved transducer, now also available on Affiniti 50—to enhance diagnostic confidence in abdominal, hepatology, and vascular and gynaecological ultrasound exams as well. The new Liver Fat Quantification tools complement the company’s existing ultimate solution for liver assessment with real-time shear wave imaging, contrast enhanced ultrasound (CEUS) and fusion and navigation. The Liver Fat Quantification tools, which feature ease of use plus intuitive workflow and reporting, is available on both the C5-1 and the small footprint mC7-2 MicroConvex transducer, to accommodate different body types, from smaller patients to high BMI patients.3
Launch of Philips’ Liver Fat Quantification Solution at RSNA 2021
Philips will debut its Liver Fat Quantification solution at the Radiological Society of North America (RSNA) Annual Meeting later this month. For more information on Philips Liver Fat Quantification, including live demos, follow @PhilipsLiveFrom for updates throughout the RSNA event. For more information on Philips’ portfolio of liver ultrasound solutions, visit the Philips liver assessment webpage, and join Philips at RSNA 2021 where the company will spotlight its latest portfolio of radiology workflow solutions and smart connected imaging systems to increase efficiency and diagnostic confidence in precision care and treatment.
References:
1 Asrani et al. Burden of liver disease in the world. J Hepatology. 2019;1.
2 Younossi ZM. Non-alcoholic fatty liver disease – A global public health perspective. J Hepatol. 2019;70(3):531-544.
3 Chen J. Realizing dramatic improvements in the efficiency, sensitivity and bandwidth of ultrasound transducers. Koninklijke Philips Electronics N.V.


 April 16, 2025
April 16, 2025 








