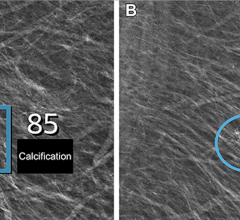
NovaRad has recently received U.S. Food and Drug Administration (FDA)clearance for reading and interpreting mammography studies for its NovaPACS which is used for the distribution and viewing of mammography studies, including window,level,zoom and volumetric measurements.
If you enjoy this content, please share it with a colleague
Related Content
July 29, 2024 — iCAD, Inc., a global leader in clinically proven AI-powered cancer detection solutions, announced a ...
Industry trade shows and conferences seem to be making their comeback in 2024. And the Healthcare Information and ...
Don't miss ITN's latest "One on One" video interview with AAWR Past President and American College of Radiology (ACR) ...
July 23, 2024 — Professional registration is open for RSNA 2024, the world’s largest radiology forum. This year’s theme ...
June 28, 2024 — Konica Minolta Healthcare Americas announced today a strategic partnership with Apollo Enterprise ...
June 27, 2024 — RamSoft, a global leader in cloud-based RIS/PACS radiology solutions, and RADPAIR, a trailblazer in ...
June 21, 2024 — Konica Minolta Healthcare Americas announced a strategic partnership with Comp-Ray, Inc., a Christie ...
June 20, 2024 — The technologically advanced VELA Mammography Chair, offered by Enable Me, a VELA Medical company ...
June 12, 2024 — Carestream launched its Image Suite MR 10 Software to help deliver a boost to productivity and ...
June 4, 2024 — Using artificial intelligence (AI), breast radiologists in Denmark have improved breast cancer screening ...


 July 29, 2024
July 29, 2024