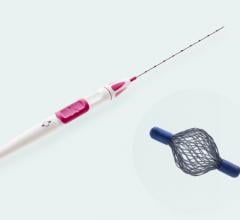
April 12, 2007 - Suros Surgical Systems, Inc. a subsidiary of women's imaging group, Hologic, Inc.announced today the commercial launch of the Suros Celero,the first U.S. FDA-cleared spring loaded, vacuum assisted core biopsy device for the breast ultrasound market.
With the option of firing inside or outside the breast, the device is designed to access hard-to-reach lesions in the axilla, near the chest wall, near implants or behind the nipple. The lightweight design of the handheld Celero and its echogenic needle reportedly provide smooth penetration to lesions while reducing deflection and offer a clearly visible aperture location for target verification under ultrasound imaging prior to tissue acquisition. Celero securely holds the tissue sample in place while acquiring large cores. The philosophy behind firing and collecting tissue in two separate steps is that it allows for confirmation.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now

 July 07, 2021
July 07, 2021