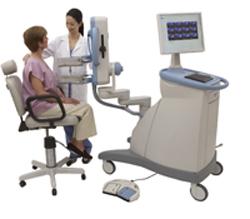
September 8, 2011 — Naviscan announced it obtained CE Mark approval for its high-resolution positron emission tomography (PET) scanner and PET-guided biopsy accessory. The breast application of the scanner is positron emission mammography (PEM). Naviscan has expanded its distribution into 13 European countries and has already received its first two orders in Germany and Switzerland.
The company manufactures the only commercially available, high-resolution PET scanner with PET-guided biopsy system. The scanner uses PET technology to produce tomographic images allowing physicians to visualize breast tumors with an unprecedented resolution of 1.6 mm, about the width of a grain of rice.
In recently published data, PEM has been found to be significantly more precise than existing technologies at identifying benign and cancerous lesions, in what scientists call "specificity." This reduces the number of unnecessary biopsies. It is a welcomed outcome for women and physicians looking to reduce patient trauma and cost associated with unnecessary procedures.
The Naviscan PET scanner is currently installed and available in breast and imaging centers throughout the United States and other parts of the world.
For more information: www.naviscan.com


 July 30, 2024
July 30, 2024 








