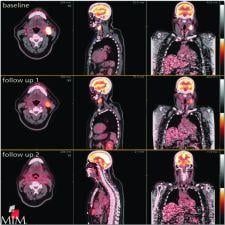
MIMvista's MIMfusion serial exam aims to eliminate labor-intensive, multistep processes such as fusion, linking and layouts. With MIMvista’s customizable workflows, any number of exams can reportedly be linked or fused automatically for easy comparison. For example, tumors may be tracked and correlated over multiple time points. Tumors may be defined with PET Edge on serial PET exams with provide quantitative analysis All ROI statistics may be exported to a spreadsheet for reportedly streamlined reporting, according to the company.


 August 01, 2022
August 01, 2022 







