Sept. 9, 2024 — Lunit has announced a collaboration with Roche to integrate Lunit SCOPE PD-L1 22C3 TPS into Roche's navify Digital Pathology platform.
This marks the first deployment of Lunit's AI technology with Roche, supporting pathologists and scientists with enhanced tools for cancer research as part of Roche's initiative to create a collaborative digital pathology ecosystem.
Roche's navify Digital Pathology is an enterprise software application designed for the pathologist's workflow. It now incorporates a range of AI-driven algorithms, including Lunit SCOPE, creating easy access to external innovation. The integrations aim to offer a comprehensive suite of digital pathology tools that enhance biomarker testing for cancer research and improve patient outcomes through precision medicine.
The collaboration spans significant global territories, starting with the U.S., with future expansion targeted for Canada, the EU (UK), Korea and Japan.
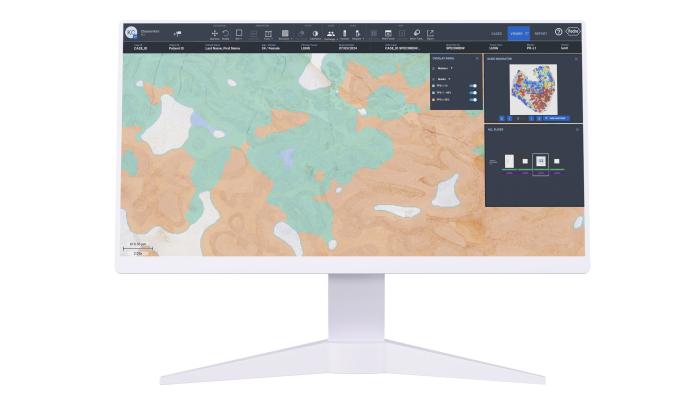
Lunit SCOPE PD-L1 22C3 TPS is an AI-powered tool that provides high-value insights and analysis of the PD-L1 tumor proportion score (TPS), a critical biomarker in cancer immunotherapy. By automating the PD-L1 scoring process, it provides more accurate, consistent, and efficient assessments of PD-L1 TPS scoring, a particularly challenging biomarker.
Lunit SCOPE PD-L1 has been available for research use only since 2021. This new accessibility in the Roche navify platform is designed to maximize access to this leading-edge AI.
"We're thrilled to work together with Roche within its open digital pathology ecosystem, which we believe will create powerful synergies and represent a significant step toward making Lunit's AI tools accessible globally," said Brandon Suh, CEO of Lunit. "Today, we have made PD-L1 analysis available, and we look forward to sharing more of our tools on this platform in the future."
Lunit SCOPE PD-L1 TPS is for research use only, and is not for use in diagnostic procedures.


 January 23, 2025
January 23, 2025