Gamma Medica Inc. will demonstrate the LumaGEM Low Dose Molecular Breast Imaging (MBI) System at RSNA 2012.
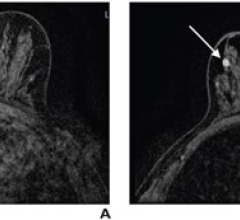
LumaGEM MBI is the first commercially available, U.S. Food and Drug Administration (FDA)-cleared, planar, dual-head, fully solid state digital imaging system utilizing cadmium zinc telluride (CZT) technology used for digital breast imaging. LumaGEM Low Dose MBI is a major advancement in the area of molecular imaging, identifying tumors in dense breast tissue that are often not visible with mammography.
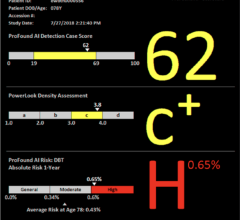
A 2011 publication in Radiology (doi:10.1148/radiol.10100625), documented the addition of molecular breast imaging (MBI) in 1,000 patients, following an injection of 20 mCi Tc-99m sestamibi, to screening mammography (MG) significantly increased detection of breast cancer in dense breasts from 27 percent with MG alone to 91 percent with the combination (p=0.016).
After implementing modifications that permit further radiation dose reduction, performance has been clinically compared between screen mammography and prevalent screen low-dose MBI in 2,400 women with dense breasts using an 8 mCi injection of Tc-99m sestamibi, dual-head CZT detectors by Gamma Medica, and acquired in dynamic frames to allow the generation of 4 mCi equivalent images.
For more information: www.gammamedica.com


 July 29, 2024
July 29, 2024