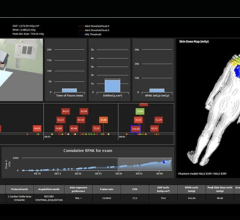
Sept. 10, 2024 – Landauer recently unveiled a package of updates to its dosimetry account management platform, myLDR. Included in the updates is Signature Suite, a compliance documentation upgrade that reduces the time, risk and effort involved with meeting regulatory requirements by enabling subscribers to leverage a completely digital workflow.
For a Nuclear Regulatory Commission (NRC) or similar inspection, organizations are required to provide inspectors with documentation that proves they are reviewing dosimetry reports, and the reports are being provided to their dosimetry participants.
“Many organizations are using cumbersome, manual processes to review and sign reports, provide them to participants, and create documentation proving their compliance,” says David Foth, Product Manager for myLDR. “myLDR Signature Suite simplifies record-keeping for compliance purposes, reduces manual activities around reporting, and saves valuable time.”
myLDR Signature Suite provides users with the ability to:
- Digitally sign all reports that require a signature
- Access a secure archive of all digitally signed reports
- Quickly publish reports to IDR (Individual Dose Review)
- Instantly notify all participants about new reports
- Keep records of participant IDR access
- Easily generate a report for inspections
myLDR Signature Suite is available as an add-on to myLDR.
For more information, please visit landauer.com.


 May 06, 2024
May 06, 2024