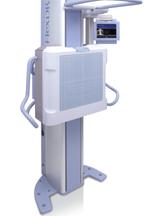
Konica Minolta Medical Imaging's FlexDR is an upright, flat panel digital radiography system.
July 23, 2009 – Konica Minolta Medical Imaging introduced today the FlexDR, an upright, flat panel digital radiography system designed for high-throughput environments.
The FDA-cleared FlexDR features an amorphous Silicon (aSi) and Cesium Iodide (CsI) digital receptor for high- quality images with minimum X-ray exposure. The product is available for immediate sale in the United States. The FlexDR enables technologists to boost productivity by utilizing the same imaging Control Station as the company’s Nano and Xpress CR systems. This creates a unified CR/DR platform that combines cassette-less and cassette based digital radiography into one cost effective, efficient workflow solution.
The sub-console mounted onto the FlexDR provides the ability for technologists to verify immediately patient information, as well as, control collimation and approve the image right at the patient’s side, creating a more efficient workflow that can also reduce both total exam time and patient call backs. The system is optimized for patient comfort and has a large vertical travel range for lower extremity imaging.
According to the manufacturer, each image is available for review approximately five seconds after exposure.
For more information: medical.konicaminolta.us.


 May 17, 2024
May 17, 2024