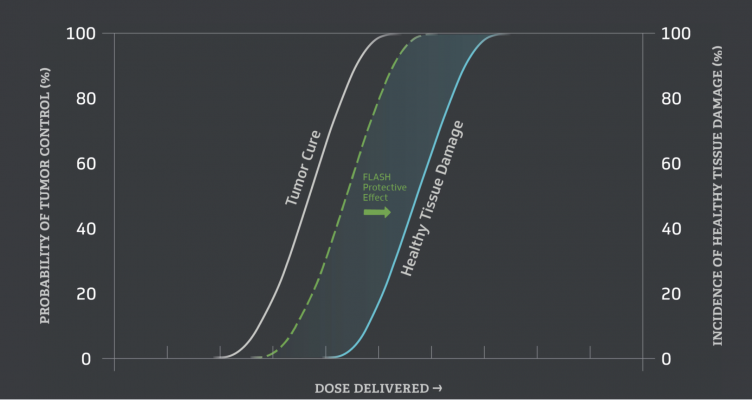
The FLASH Effect significantly improves the therapeutic ratio for curing cancer
July 28, 2021 — IntraOp Medical Corporation announced that Lausanne University Hospital (CHUV, Switzerland) enrolled the first patients in the Impulse Trial: A phase I dose-escalation study of high dose rate radiotherapy with electrons in patients with skin metastases from melanoma. The trial is a key milestone for the groundbreaking research collaboration agreement between IntraOp and the CHUV, executed in 2020. The Impulse Trial is the first in the world to evaluate the potential of leveraging the biological phenomenon known as the "FLASH Effect" to provide radiotherapy with curative intent to radio-resistant cancers.
To conduct the study, researchers will utilize the IntraOp Mobetron platform modified to deliver precision electron beam therapy at high dose rates to produce the FLASH effect. "Leveraging their vast experience studying FLASH with an experimental LINAC, the CHUV's researchers have diligently worked through the past year to prepare the FLASH capability of the Mobetron for biological and now human clinical work," stated Kenneth Brooks, Ph.D. Vice President of Sales and Corporate Strategy at IntraOp. "With its fine beam structure control capabilities now validated, the Mobetron is the clear choice for researchers who, as those of the CHUV, are looking to explore possible effects of changing fine structure on the basic science that underlies this biologic effect and thus expands their institution's research capabilities into FLASH with a clear path to clinical translation."
Scientists and clinician-researchers from CHUV are world-renowned pioneers in the characterization of the FLASH effect, having published numerous groundbreaking studies in animal testing as well as the first human treatment. In preclinical studies, the FLASH effect has demonstrated several biological benefits and an improved therapeutic index by producing a protective effect for normal tissue at certain ultra-high dose rates and volumes. Professor Jean Bourhis, head of radiation oncology and principal investigator noted: "The Impulse Trial is the first in a series of clinical trials we have outlined to better understand the potential of the FLASH effect. Melanoma is one of the most radio-resistant cancers and curative therapy with conventional radiotherapy is rarely possible. By leveraging the FLASH effect, we may be able to optimize the therapeutic ratio and escalate the irradiation dose to completely kill cancer cells with a protective effect on normal tissue."
"The Impulse trial is a major step forward for FLASH research and we congratulate Prof. Bourhis, Dr. Vozenin, Dr. Moeckli, and the rest of the team at CHUV in their ongoing achievements to realize this breakthrough," said Derek T. DeScioli, Chief Executive Officer of IntraOp. "With our advanced electron therapy technology, commitment to patient-driven innovation, and growing cohort of research collaborators we are excited to be leading this new frontier in radiation oncology," added Mr. DeScioli.
Note: IntraOp's High Dose Rate (HDR) functionality is for investigational use only and is not approved for routine clinical use.
For more information: www.intraop.com


 July 31, 2024
July 31, 2024 








