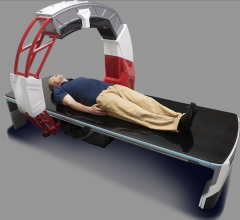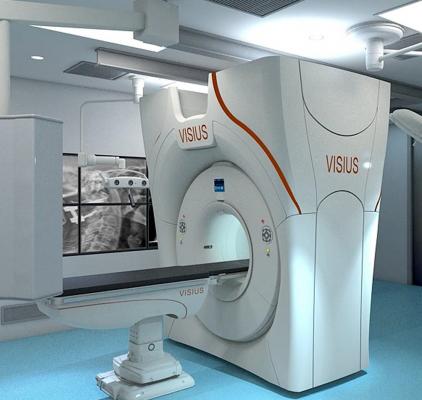
July 23, 2013 — IMRIS has announced U.S. Food and Drug Administration (FDA) 510(k) clearance to market VISIUS iCT, the first and only ceiling-mounted intraoperative computed tomography (iCT) on the market.
Visius iCT provides personalized radiation dose management together with diagnostic quality imaging during the surgical procedure to assist surgeons in critical decision making.
The 64-slice scanner moves into and out of the operating room during surgery using ceiling-mounted rails to ease workflow. This enables multiple room configurations to meet both clinical requirements and increase utilization without compromising image quality or exam speed.
Patient transport and the need for floor-mounted rails used in other systems is eliminated, which opens up valuable operating room (OR) space and allows unimpeded movement of surgical equipment and simplified infection control. The system also offers the longest scanner travel range on the market today.
In addition, Visius iCT features a suite of software applications such as 3-D volume rendering to aid in surgical planning and dose reduction which considers each patient's unique characteristics and needs to maximize image quality and minimize dose. The system software allows healthcare practitioners to visualize dosage prior to scan and adjust settings based on the specific clinical need with detailed dosage reports produced after each scan.
For more information: www.imris.com


 August 09, 2024
August 09, 2024