November 6, 2007 - ImaRx Therapeutics Inc. and Royal Philips Electronics announced a research collaboration to evaluate Philips ultrasound technology as part of ImaRx’s SonoLysis program to develop new treatment for acute ischemic stroke, aiming to determine the optimal ultrasound parameters to use with ImaRx’s proprietary MRX-801 microbubble technology.
Under the agreement, Philips’ Medical Systems division will provide ultrasound devices and technical assistance to ImaRx during laboratory and preclinical studies. The agreement includes a mutual exclusivity clause during the term of the collaboration. Following completion of the research program, Philips and ImaRx will have an exclusive negotiation period to discuss future development and commercialization.
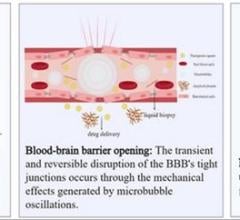
ImaRx’ SonoLysis program is focused on the development of product candidates that involve the administration of its proprietary MRX-801 microbubbles and ultrasound to break up blood clots and restore blood flow to oxygen deprived tissues. The sub-micron size of MRX-801 microbubbles may allow them to penetrate a blood clot, so that when ultrasound is applied their expansion and contraction, or cavitation, can break the clot into very small particles. ImaRx is conducting an ongoing Phase I/II multinational clinical trial evaluating its SonoLysis tPA therapy to treat patients with acute ischemic stroke.
For more information: www.philips.com and www.imarx.com


 March 19, 2025
March 19, 2025