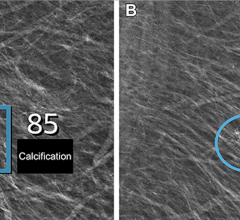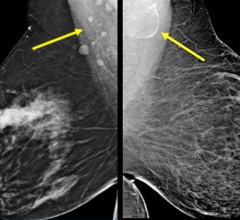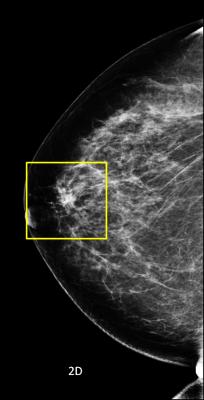
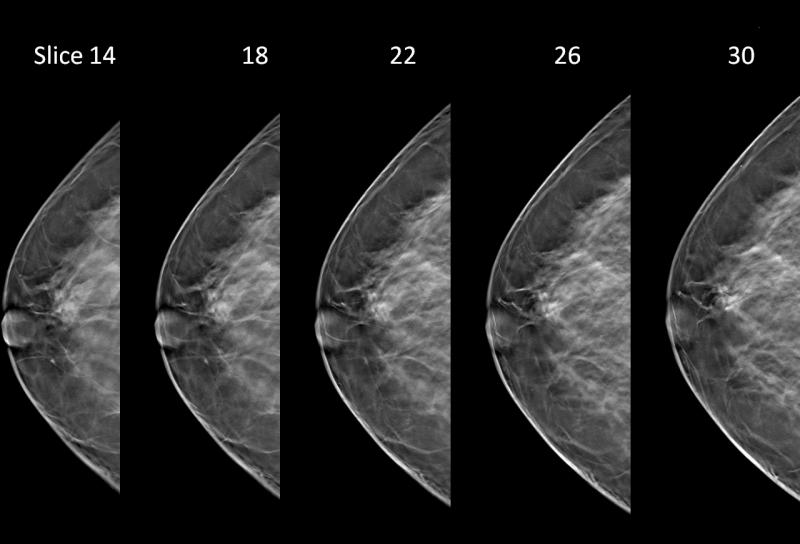
In the 2D image on we are looking at a potential lesion in the subareolar region of the breast.
December 4, 2012 — Hologic Inc. featured its newest women's health products at the 98th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) Nov. 25 through 29.
The RSNA is an international society of radiologists, medical physicists and other medical professionals with more than 50,000 members across the globe. RSNA hosts the largest medical meeting in the United States, drawing 60,000 attendees annually. The theme for RSNA's 98th Scientific Assembly and Annual Meeting was "Patients First."
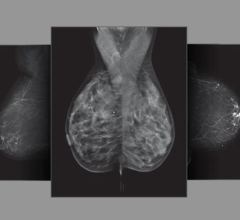
Prominently featured at RSNA this year is Hologic's Selenia Dimensions 3-D mammography (breast tomosynthesis) system. The technology was approved by the U.S. Food and Drug Administration (FDA) in February 2011 and has been available in countries recognizing the CE mark since 2008. Hologic's 3-D mammography technology is available in 47 states in the United States and 28 other countries.
Hologic's 3-D mammography system placed first in the digital mammography category in the KLAS 2012 annual survey of healthcare executives and clinicians. KLAS is a research firm specializing in monitoring and reporting on the performance of healthcare vendors. 3-D mammography was recently selected by AuntMinnie as the "hottest clinical procedure" of the year for the third consecutive year. AuntMinnie is the largest and most comprehensive Web site for medical imaging professionals worldwide. In October, the Cleveland Clinic picked 3-D mammography as one of the Top 10 Medical Innovations for 2013 at the Clinic's 10th annual Medical Innovation Summit.
Fifteen of the clinical papers and over 50 of the workshops, seminars, poster sessions and scientific papers presented at the conference were on 3-D mammography. Clinical presentations cover the use of tomosynthesis in screening and diagnostic applications.
Hologic offered training sessions including hands-on experience reading 3-D breast tomosynthesis images in combination with conventional and synthesized 2-D images. Brief lectures will provide an overview of the technologies prior to the hands-on sessions. Each program will be taught by a leading breast imaging radiologist. Radiologists can register for a workshop through the RSNA meeting web site.
Some of Hologic's other advances in breast tomosynthesis on display at the conference included its tomosynthesis biopsy option for use with the Affirm biopsy guidance system and its C-View synthesized 2-D image option. The tomosynthesis biopsy option is CE marked and is pending FDA clearance. C-View images are designed to be used with tomosynthesis in the screening and diagnosis of breast cancer, eliminating the need for a separate 2-D exposure. The C-View synthesized 2-D image option received CE marking in 2011 and while not yet approved in the United States, received a favorable vote from an FDA Advisory Panel in October 2012.
Other Hologic Product Advances Highlighted This Year
Selenia and Selenia Dimensions 2-D mammography systems continue to be recognized as the standard-bearers for the industry, ranking second and third, respectively, in the digital mammography category in the KLAS 2012 annual survey of healthcare executives and clinicians.
New this year is the Selenia Dimensions 2-D Screening Package, an elegant solution for customers seeking a cost-effective addition to their breast cancer screening capabilities. The Dimensions 2-D Screening Package can be upgraded quickly and efficiently to include diagnostic, interventional, or tomosynthesis capabilities, giving facilities unlimited flexibility in expanding service offerings at a time of their choosing.
Also on display this year is Hologic's Selenia Dimensions Contrast Enhanced 2-D Imaging, an effective means of adding physiological information to a diagnostic exam. This option is CE marked and pending FDA clearance.
Hologic's Image Analytics innovations include Quantra breast density assessment software, designed to provide a BI-RADS-like value for the consistent reporting of breast composition and Hologic's BACS (Breast Arterial Calcification Scoring) software package. BACS can be used to evaluate calcified plaques in arteries. Quantra is CE marked and cleared for sale in the U.S. BACS is CE marked and pending FDA approval.
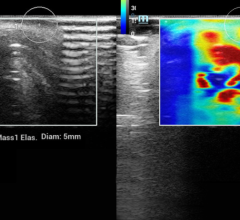
In the biopsy area Hologic is premiering a new introducer for use with the ATEC biopsy system under ultrasound guidance. One insertion allows for both biopsy needle placement and marker deployment.
In breast magnetic resonance imaging (MRI), Hologic is showing a new 16-channel Sentinelle Breast Coil array designed to provide excellent signal-to-noise ratio as well as optimal access for breast biopsies.
In Skeletal Health, Hologic is highlighting two products. The first is a new Advanced Body Composition Assessment package on the Discovery platform. This package is designed to assist clinicians with the diagnosis and monitoring of obesity related metabolic diseases, athletic training regimens and sports injury rehabilitation programs. The second, shown for the first time at RSNA as a commercial product, is Hologic's Fluoroscan InSight-FD Mini C-arm with flat panel detector technology. The product is designed specifically for orthopedic and extremity surgeons and offers a thin profile and improved workspace access with enhanced ease of positioning.
For more information: www.hologic.com


 July 29, 2024
July 29, 2024