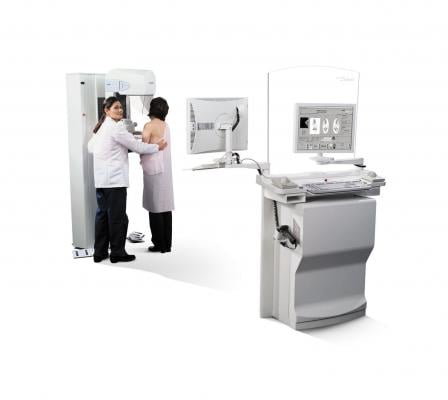
Hologic will feature its Selenia and Selenia Dimensions 2D mammography systems at RSNA, which continue to be recognized as the standard-bearers for the industry, ranking second and third, respectively, in the digital mammography category in the KLAS 2012 annual survey of healthcare executives and clinicians.
New this year is the Selenia Dimensions 2D Screening Package, an elegant solution for customers seeking a cost-effective addition to their breast cancer screening capabilities. The Dimensions 2D Screening Package can be upgraded quickly and efficiently to include diagnostic, interventional, or tomosynthesis capabilities, giving facilities unlimited flexibility in expanding service offerings at a time of their choosing.
Also on display this year is Hologic’s Selenia Dimensions 2D Contrast Imaging Option, an effective means of adding physiological information to a diagnostic exam. This option is CE marked and pending FDA clearance.
Hologic’s Image Analytics innovations include Quantra breast density assessment software, which now can provide a BI-RADS-like value for the consistent reporting of breast composition and Hologic’s BACS (Breast Arterial Calcification Scoring) software package. BACS can be used to evaluate calcified plaques in arteries. Quantra is CE marked and cleared for sale in the U.S. BACS is CE marked and pending FDA approval.
In the biopsy area Hologic is introducing a new introducer for use with the ATEC® biopsy system under ultrasound guidance. One insertion allows for both biopsy needle placement and marker deployment.
For more information: www.hologic.com


 December 08, 2025
December 08, 2025 








