HealthMyne, an imaging informatics company, announced 510(k) clearance from the U.S. Food and Drug Administration (FDA). The new analytics software package is designed to provide radiologists with significant workflow efficiencies related to oncology cases, as well as advanced decision support.
New image characterization and tracking features are now provided in a comprehensive, quantitative imaging dashboard, which includes time-sequenced Epic EHR (Electronic Health Record) information. The analytics software also offers convenient tracking of a nodule or tumor over multiple, spatially registered imaging studies. This can reveal crucial information about whether the structure is growing, shrinking, or stable.
Structure change can be particularly useful in monitoring and/or adjusting a patient’s treatment regime. Importantly, with cancer treatment options continuing to increase in both number and specificity, the HealthMyne software can provide valuable quantitative insight for improved clinical decision-making.
Cancer screening programs can also benefit from nodule characterization. As an example, nodule growth is automatically identified by HealthMyne software for patients participating in lung cancer screening programs. This can be an indicator of potential nodule malignancy, and therefore, suggest extra scrutiny.
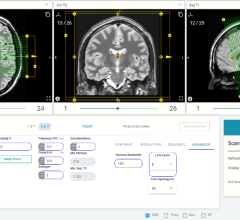
“By automatically delineating the boundaries of a lung nodule (or tumor), our software can make other sophisticated measurements within those boundaries - such as quantitatively describing important properties like size, shape, and texture,” stated Roger Chylla, CTO for HealthMyne. “Physicians can examine these properties to make critical judgements about a patient’s prognosis, follow-up schedule, or how to best treat them.”
For more information: www.healthymyne.com

 January 15, 2024
January 15, 2024