Discovery PET/CT 600
May 29, 2009 - GE Healthcare will showcase a broad range of technological evolutions in imaging, such as PET VCAR, FASTlab, CT, PET/CT and MR systems and more at the 45th annual meeting of the American Society of Clinical Oncologists (ASCO), May 29- June 2 in Orlando, Fla.
GE will demonstrate its breadth in oncology technology, from treatment monitoring to productivity enhancing IT solutions. Visitors to the GE booth will see new advances in molecular imaging technologies such as the Positron Emission Tomography/Volume Computer Assisted Reading (PET-VCAR) and FASTlab automated PET radiopharmacy through innovative volume navigation ultrasound and cell imaging.
Some of the products on display include GE Healthcare's PET VCAR, which provides clinicians the opportunity to manage their PET studies with efficiency, compare multiple exams with precision, and report with confidence. The volume PET/CT application is optimized for visualization and analytical monitoring of cancer disease progression, or tumor response to therapy. Enabling early quantification and evaluation of treatment effectiveness, it is a valuable tool for treatment planning. It features workflow enhancements for both single and multi-exam review, and includes exam-to-exam auto-registration; tumor segmentation and quantification; and multiplanar image review.
GE Healthcare provides complete solutions for molecular imaging, from imaging agents to imaging systems. With a full range of radiopharmaceutical equipment, clinicians have the ability to set up customized PET production units. FASTlab is the solution to the ever-evolving challenges of tracer production. It is designed to accommodate different chemistries, facilitating the production of multiple PET tracers such as fludeoxyglucose ([F-18]FDG) and sodium fluoride ([F-18]NaF). FASTlab features a cassette that incorporates premeasured and preloaded reagents. It has the capacity to deliver higher yields at a very low variation, improved reproducibility and fast synthesis.
GE Healthcare’s multimodality imaging systems (CT, PET/CT and MR) are designed specifically for radiation oncology. They provide the precision and accuracy required to take full advantage of today's advanced treatment techniques to help ensure clinical confidence.
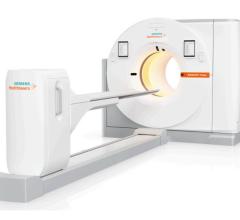
GE Healthcares Discovery PET/CT 600 scanner combines advanced molecular imaging tools with diagnostic CT in one scanner, helping doctors with earlier diagnosis, accurate tumor localization and assessment of how well their patient is responding to treatment.
On the Discovery PET/CT 600 scanner, GE’s breakthrough MotionFree PET/CT imaging technologies are integrated with VUE Point HD high definition intelligent reconstruction technique. Combined, these technologies can help doctors improve small lesion detection, image quality, and quantitative accuracy while addressing the biggest clinical challenge in PET/CT; motion.
With the introduction of the new MR Oncology Package, GE brings the benefits of magnetic resonance imaging for today’s advanced radiation therapy. The package consists of a detachable flat-surface patient table, flexible surface coils for high-resolution imaging and treatment positioning aids. For sites with existing MR or those needing to expand services from diagnostics to radiation therapy, the MR Oncology package provides the perfect solution. This exclusive system is easy to use and adds capability that helps drive productivity across multiple clinical areas.
GE will also showcase the IN Cell Analyzer 2000, a flexible cell imaging system for high content analysis with excellent image quality, speed and ease-of-use for all screening and research needs. The flexibility of the system enables scientists to perform a wide variety of previously challenging experiments with a single instrument: from investigative microscopy through to automated screening, and imaging of organelles, cells, tissues and whole organisms.
The IN Cell Analyzer 2000 has a unique combination of hardware and software features for extremely fast image acquisition making it ideal for screening. The robust construction of the instrument (designed using Six Sigma principles) ensures its reliability for high throughput use when carrying out demanding applications in a multiuser environment.
The system has a number of new enabling features, including rapid preview scanning of a selected area of a sample at any available magnification prior to starting an acquisition run. It also has a large chip CCD camera coupled with a widefield illumination source that is twice as bright as a conventional xenon lamp. It also offers whole-well imaging to capture an entire well in a single image and a wide range of objectives (2x – 100x) to suit a variety of sensitivity requirements.
GE will feature its recently launched ultrasound system, the LOGIQ E9, for radiology and vascular applications that fuses ultrasound images with images from other imaging modalities like CT and MR. The LOGIQ E9 offers (the feature is an option not included) volume navigation, an innovative tool that incorporates two key components to maximize the system’s new agile ultrasound architecture: ‘Fusion’ to combine the advantages of real-time ultrasound imaging with the high spatial and contrast resolution of CT, MR or PET; and a ‘GPS-like technology’ to track and mark a patient’s anatomy during the ultrasound exam, bringing confidence and productivity to both diagnostic and interventional studies.
Visitors to the GE Healthcare booth will find exciting information about GE's latest molecular imaging agent, Adreview (Iobenguane I 123). It is indicated for use in the detection of primary or metastatic pheochromocytomas and neuroblastomas, as an adjunct to other diagnostic tests. AdreView is the only FDA-approved Iodine-123 meta-iodobenzylguanidine (I 123 mIBG) agent for the imaging of these rare neuroendocrine tumors. AdreView allows you the use of planar or single photon emission computed tomography (SPECT) imaging which may help enhance image interpretation and tumor definition.
Also featured in the GE booth at ASCO is Cytori’s StemSource technology. The two companies recently announced an agreement by which GE Healthcare will commercialize Cytori’s StemSource technology in the North American stem cell banking and research markets. The StemSource technology includes automated equipment to process stem and regenerative cells found in adipose tissue, cryopreserve them or use them directly for research purposes.
In January 2009, Cytori and GE Healthcare formed a separate agreement to commercialize Cytori’s products in 10 European countries. This includes selling the Celution 800/CRS System in the European cosmetic and reconstructive surgery market as well as selling StemSource products in the European cell banking and research markets.
The April partnership agreement is similar in nature to the European agreement, but is limited to the sale of StemSource banking and research products in the U.S., Canada and Mexico for 18 months starting in the second quarter of 2009. Today’s agreement does not include U.S. commercialization of Cytori’s Celution System, which is currently under review by the FDA.
For more information: www.gehealthcare.com


 July 02, 2024
July 02, 2024