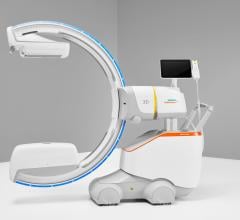
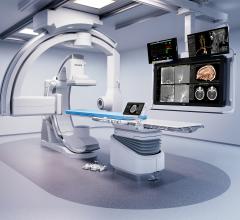
November 28, 2011 — At RSNA 2011, GE Healthcare unveiled the Discovery IGS 730, the first interventional X-ray system designed to capture the advantages of both floor- and ceiling- mounted systems. With laser-guided motion technology on a motorized mobile gantry for predictable and precise trajectories, Wide Bore 3-D for ease in 3-D acquisitions and more than 20 advanced applications available, the Discovery IGS 730 is positioned for a new era of interventional procedures. The significance of this innovation to interventional imaging has been compared to that of the invention of flat panel technology.
“Our goal was to pioneer a solution that eliminates the historical trade-offs our customers had to make when selecting from the interventional imaging systems. In general, the trade-off between ceiling- and floor-mounted systems means compromising patient access for room airflow and sterility, while the choice between fixed units and mobile C-arms means compromising image quality for mobility. Now interventionalists and surgeons who perform complex, minimally invasive procedures have an entirely new category of imaging system that eliminates the need to compromise: Discovery. At GE Healthcare, we believe the Discovery IGS 730 has the potential to revolutionize the field of interventional imaging,” said Hooman Hakami, president and CEO of GE Healthcare Interventional Systems.
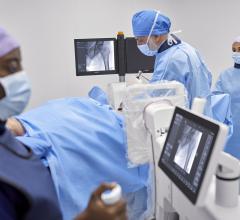
The Discovery IGS 730 is a revolution in imaging. It is neither floor- nor ceiling-mounted, but enables full patient access without the need to suspend the system above the patient. It has the mobility of a C-arm with the power and image quality of a fixed system. This laser-guided, motorized mobile gantry creates an interventional environment without boundaries. It allows complete access to the patient and unlimited parking capability, while creating sterility for a flexible and secure operating room (OR) environment. The unique gantry comes with a new wide bore design, which allows for steep angles, ease in 3-D acquisition, especially for large patients.
The Discovery IGS 730 C-arm is mounted on an advanced guided vehicle (AGV), a motorized and fully mobile system. Based on laser guidance, the AGV can move freely from imaging position to parking or back- out positions, using predefined trajectories to provide total patient access. The Discovery IGS 730 features “One-Touch-Back-Out,” enabling fast and easy gantry movement away from the patient. The motion is predictable, precise and easy to use, allowing fine control and positioning at any moment in the procedure. Parking locations and back-out distances are customizable for different room configurations.
“My first impression was that this system will allow us to have levels of access to patients in ways we have never had before. This particular unit removes the limitations of both ceiling- and floor-mounted systems without losing any of the advantages,” said Hal Folander, M.D., chairman of the Radiology Department and Section Chief of Interventional Radiology at St. Luke's Hospital and Health Network in Pennsylvania, who has participated in clinical simulations on phantoms using the Discovery IGS 730. “Having been involved in research and development of this type of product for 25 years, I would say this is, probably, one of the most revolutionary interventional projects that I’ve seen. The innovation will be equivalent to or greater than the invention of flat panel technology for interventional procedures.”
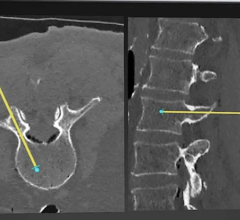
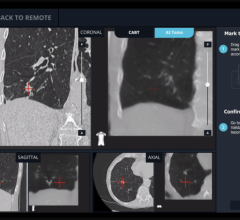
In addition to being mobile, Discovery IGS 730 comes equipped with more than 20 advanced applications. One example is the new Wide Bore 3-D application for ease in 3-D acquisitions and the ability to allow off-centered 3-D acquisitions to cover anatomies such as the borders of the liver during liver oncology procedures or the skin line during image-guided needle procedures.
“With Discovery IGS 730, doctors will be able to rediscover space and movement while accessing all the advanced imaging capabilities of GE premium interventional systems,” said Chantal Le Chat, general manager of GE Healthcare Interventional Radiology.
The system does not yet have regulatory clearance in either Europe of the United States.
For more information: www.gehealthcare.com

 June 19, 2024
June 19, 2024