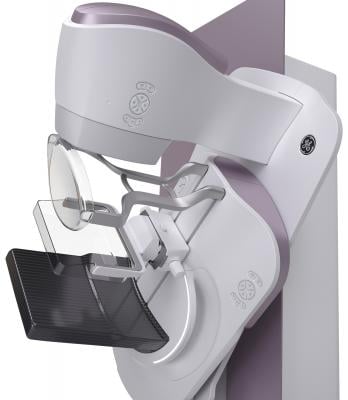
March 24, 2017 — GE Healthcare announced the launch of its new Senographe Pristina mammography system, designed to make breast screening more comfortable and inviting for patients.
A recent study conducted by Evans, et.al, found that painful exams explain why 25 to 46 percent1 of women failed to return for further breast imaging. The study concluded that effective pain-reducing interventions in mammography are needed to encourage more women to come in for screening. The target participation rate in Canadian organized breast cancer screening programs is 70 per cent of eligible women2. Currently all provincial programs fall below this rate2.
Senographe Pristina features a range of ergonomic features that are intentionally designed to enable patients to be more comfortable. All parts in contact with the patient's breasts, for example, have gentle, rounded corners for greater comfort. The system features comfortable armrests that relax the pectoral muscles to simplify positioning, compression and image acquisition.
Traditional mammography systems compress the breast automatically, which can be a source of considerable discomfort. Senographe Pristina features a self-compression tool that helps give women a sense of control by allowing them to manually adjust the degree of breast compression. Under the direction of a technologist, the patient can set compression to a level that feels right for them.
Taken together, these design features mean that technologists are better able to focus on precise positioning, making exams easier and faster. Poor positioning is the cause of most clinical image deficiencies that often require a rescan3.
For more information: www.gehealthcare.com
References
1. Whelehan P, Evans A, Wells M, Macgillivray S. The effect of mammography pain on repeat participation in breast cancer screening: a systematic review. Breast. 2013; 22(4):389–94.
2. Cancer Screening in Canada: An Overview of Screening Participation for Breast, Cervical and Colorectal Cancer, http://www.partnershipagainstcancer.ca
3. U.S. Food and Drug Administration http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm495378.htm


 July 29, 2024
July 29, 2024 








