GE Healthcare Announces CE Marking for Its Breast Tomosynthesis Solution, SenoClaire
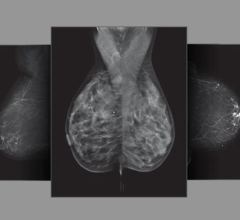
July 23, 2013 — GE Healthcare, hailed a new era for breast imaging today as it announced CE marking for SenoClaire, GE’s new generation of breast tomosynthesis solution designed with a three-dimensional imaging technology. Powered by ASiRDBT, SenoClaire technology uses a low-dose short X-ray sweep around the positioned breast with nine exposures acquired with a “step-and-shoot” method, removing the potential motion from images.
“Our goal was to pioneer an upgrade solution for both our digital mammography platforms, Senographe Essential and Senographe Care, providing flexibility for customers to enhance their clinical offering for their clinic and patient workflow,” said Prahlad Singh, general manager, Women’s Health, GE Healthcare — Detection and Guidance Solutions (DGS).
More precision, More confidence, Less dose: SenoClaire2
With GE’s SenoClaire, powered by ASiRDBT a single MLO view provides clinical non-inferiority compared to a two-view digital mammography exam — at half the dose and with just one compression. Subsequently, this solution has the potential to replace digital mammography exams in screening to help radiologists detect breast cancer.
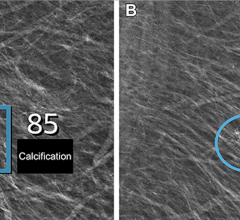
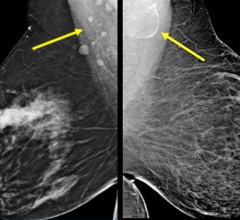
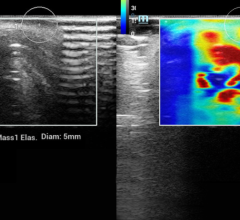
In addition, SenoClaire will help deliver superior sensitivity for architectural distortions and masses, help improve specificity of lesion margin visibility and ultimately, help radiologists reduce recall rates through better characterization of findings. Finally, its images are compatible with major picture archive and communication system (PACS), so that radiologists can integrate them easily into their environment and get the most from their investment.
First shipments have started in Europe, Middle East, Australia and Latin America with a solid flow of customer sites placing orders.
SenoClaire is not FDA approved. SenoClaire may not be available in all regions.
For more information: www3.gehealthcare.com


 July 29, 2024
July 29, 2024