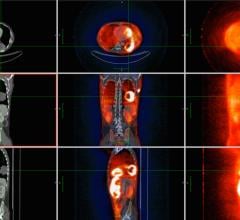
The FDA has granted clearance for GE Healthcare’s newest positron emission tomography/computed tomography (PET/CT) scanner, which is part of GE’s Discovery family of scanners designed to enable earlier detection and monitoring of disease with advanced molecular imaging technology in both hardware and software.
The new system will be optimized for use in oncology, which represents more than 90 percent of clinical PET/CT exams. The system continues using high-sensitivity crystals, along with GE’s exclusive VUE Point HD, high definition imaging to help clinicians advance toward the goal of motion free PET/CT imaging.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now


 July 02, 2024
July 02, 2024