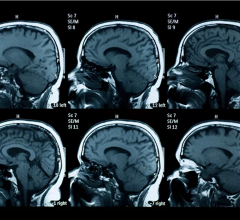
July 5, 2007 –The U. S. Food and Drug Administration recently warned that a commonly used contrast agent used to improve MRI images has been linked to a potentially fatal disease and patients who have had an MRI Scan and been diagnosed with NSF/ NFD should review the latest FDA warnings, and Injury Claim Filing information at the NSF/NFD MRI Website.
Gadolinium-based contrast agents are commonly used to improve the visibility of internal structures when patients undergo an MRI. These contrast agents have been linked to nephrogenic systemic fibrosis (NSF) and nephrogenic fibrosing dermopathy (NFD). These potentially fatal diseases cause thickening of the skin and connective tissues that inhibits their ability to move and may result in broken bones. Other organs are at risk of thickening as well.
The Johnson Law Firm, a law firm that represents clients nationwide in cases against the manufacturers of defective medical products, announced that it has created an informational website for patients who have been diagnosed with NSF/NFD: http://www.lawyersforclients.com/nsf-nfd/index.php.
According to the FDA, patients with severe kidney insufficiency who receive gadolinium-based agents are at risk for developing the debilitating and a potentially fatal disease, NSF. Reports have identified the development of NSF following single and multiple administrations of the gadolinium-based contrast agents. In addition, patients just before or just after liver transplantation, or those with chronic liver disease, are also at risk for developing NSF if they are experiencing kidney insufficiency of any severity. FDA first notified health care professionals and the public about the gadolinium-related risks for NSF in June 2006.
For more information: http://www.lawyersforclients.com/nsf-nfd/index.php


 July 09, 2024
July 09, 2024