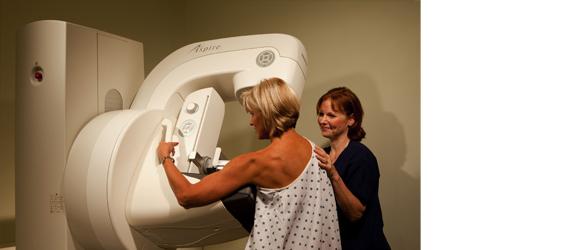
September 12, 2011 – Breast imaging facilities looking for increased diagnostic confidence in mammography screening can now benefit from the exceptional image quality of the Aspire HD full-field digital mammography (FFDM) system from Fujifilm Medical Systems U.S.A. Now U.S. Food and Drug Administration (FDA)-510(k) cleared, the Aspire HD provides image clarity that enables extraordinary detail of potential abnormalities that not only assists in more accurate and reliable diagnoses, but also results in enhanced clinician productivity.
The exceptional image quality of the Aspire HD is made possible in part by the system’s unique proprietary detector that uses innovative dual-layer amorphous selenium (a-Se) and the first use of Fujifilm’s innovative direct optical switching (DOS) technology. This new technology combined with the company’s renowned 50-micron resolution provides facilities with the confidence they need for the detailed visualization that’s required in mammography.
The new DOS technology eliminates the use of thin film transistors (TFTs), which are currently used in typical FFDM detector systems. By eliminating the TFTs, this new technology creates a direct image transfer that results in a higher efficiency image capture. This process produces images with less noise and the potential to reduce dose, while delivering the performance and reliability digital mammography users have come to expect from Fujifilm.
Fujifilm has more than 8000 FFDM systems installed worldwide. The company was the first to bring computed radiography (CR)-based FFDM to the U.S. market in 2006 and has been successfully serving the needs of hospitals and imaging centers with the Aspire ClearView-CSm and the Aspire ClearView-1m, multi- and single-imaging plate systems with Image Intelligence, Fujifilm’s proprietary image processing technology.
For more information: www.Aspire.fujimed.com


 July 29, 2024
July 29, 2024