February 2, 2016 — Focal Healthcare Inc. announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for their targeted prostate biopsy device, Fusion Bx. With the availability of Fusion Bx, clinicians are no longer limited to the traditional systematic biopsy approach for diagnosis, but can instead accurately take targeted biopsies directly from suspicious cancer regions within the prostate using magnetic resonance imaging (MRI)-ultrasound fusion technology.
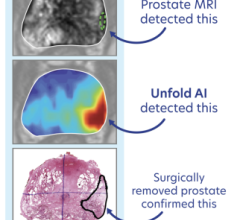
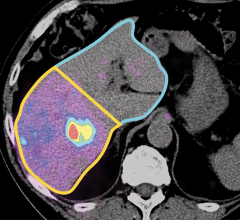
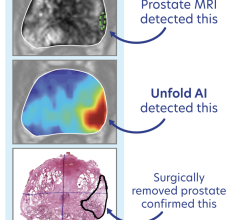
MRI has the ability to see tumors in the prostate that ultrasound alone cannot. Thus, by fusing previously acquired MR images to live ultrasound, clinicians can target suspicious tumors when taking biopsy samples. Fusion biopsy has been shown to detect high-grade cancer in 30 percent more men compared to traditional systematic biopsy. In addition, by accurately determining the location and extent of the cancer, this procedure allows clinicians to confidently monitor cancer progression over time and prescribe the appropriate course of treatment to patients with varying grades of cancer.
With this clearance, Focal Healthcare plans to make this procedure accessible to all patients.
The device has been developed in close collaboration with clinicians including Winston Barzell, M.D., Florida State University (Sarasota); Daniel Margolis, M.D., David Geffen School of Medicine at UCLA (Los Angeles); and Michael McGuire, M.D., NorthShore University Health System (Chicago). Barzell is currently conducting a clinical trial with Fusion Bx at 21st Century Oncology in Sarasota, Florida.
"In contrast to systematic 'blind' biopsies, targeted fusion biopsies reliably identify clinically significant cancers, while reducing the random detection of insignificant cancers that are frequently at risk of over-treatment. Fusion Bx is an exciting, innovative technology that will provide busy clinicians access to a state-of-the-art, user-friendly fusion biopsy system which can be seamlessly introduced to the routine urology office workflow," said Barzell
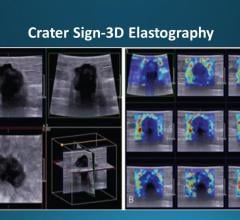
Fusion Bx is FDA 510k cleared for use by physicians for enhanced visualization of ultrasound imaging of the prostate in clinic and hospital settings. It provides 2-D and 3-D image visualization including review, manipulation and analysis tools.
For more information: www.focalhealthcare.com


 July 24, 2024
July 24, 2024