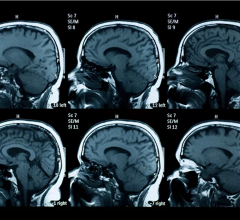
May 24, 2007 - The U.S. Food and Drug Administration (FDA) has recently asked manufacturers to include a new boxed warning on the product labeling of all gadolinium-based contrast agents which are used to enhance the quality of magnetic resonance imaging (MRI).
The requested warning would state that patients with severe kidney insufficiency who receive gadolinium-based agents are at risk for developing a debilitating and a potentially fatal disease known as nephrogenic systemic fibrosis (NSF). In addition, it would state that patients just before or just after liver transplantation, or those with chronic liver disease, are also at risk for developing NSF if they are experiencing kidney insufficiency of any severity.
FDA first notified healthcare professionals and the public about the gadolinium-related risks for NSF in June 2006. Information on the risks was updated in December 2006.


 July 09, 2024
July 09, 2024