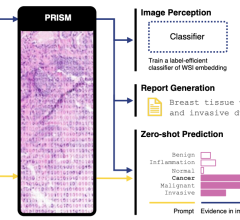
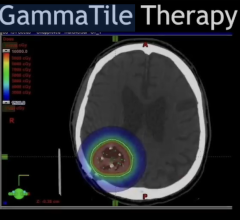
December 15, 2009 - The New FDA guidelines on current good manufacturing practices (cGMPs) for the production of positron emission tomography (PET) drugs require all PET drug manufacturers to submit a new drug application (NDA) or abbreviated new drug application (aNDA) for all PET drug products in routine clinical use by the date of implementation.
The cGMP guidelines, which will take effect December 11, 2011, are designed to ensure that PET drugs meet all requirements of safety, identity, strength, quality and purity. In the interim, U.S. facilities must continue to comply with U.S. Phamacopeia , which sets standards for the production of PET drugs.
The Society of Nuclear Medicine (SNM) worked in concert with other medical organizations to provide FDA input and review on the cGMP guidelines. Michael M. Graham, Ph.D., M.D., president of SNM and director of nuclear medicine at the University of Iowa Carver College of Medicine noted called it a major step forward as a well-defined structure in place benefits manufacturers, physicians and patients by ensuring the highest quality drugs possible.
Through its Clinical Trials Network, SNM will offer educational programs on the new regulation. Representatives from FDA will discuss the new guidelines with the molecular imaging and manufacturing community at two upcoming events. FDA representatives will present a special session during the Clinical Trials Network Workshop, which will be held Feb. 1–2, 2010, at SNM’s Conjoint Mid-Winter Meetings in Albuquerque, N.M. In addition, FDA representatives will present a half-day workshop at SNM’s Annual Meeting, June 5–9, 2010, in Salt Lake City, Utah. To date, more than 130 manufacturing sites have registered with the Clinical Trials Network.
For more information: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidan… or www.snm.org


 July 30, 2024
July 30, 2024