June 11, 2014 — VuCOMP Inc. announced it has received U.S. Food and Drug Administration (FDA) approval for M-Vu CAD for mammography version 3.1. Concurrently, VuCOMP is releasing an update to its CAD (computer-aided detection) station, which includes the ability to process additional standard screening views. Together, the CAD system updates provide radiologists with faster processing time, enhanced workflow and improved parallel architecture.
Jim Pike, president and CTO of VuCOMP, said, “We are committed to the continuous improvement of our product line, and each new release of M-Vu CAD offers advances that provide substantial value to the system and our customers. These latest updates are no exception.”
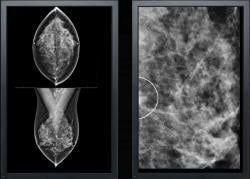
The M-Vu CAD system uses advanced computer vision algorithms to identify areas of a mammogram that are consistent with breast cancer. M-Vu CAD version 1 was the first product to meet the FDA-recommended reader study standard for proving the effectiveness of mammography CAD. VuCOMP continues to provide systematic product updates, fulfilling the company’s commitment to ongoing enhancements for its customers at no additional cost.
M-Vu CAD is approved for digital mammography systems manufactured by Carestream, Fuji, GE, Giotto, Hologic, Konica Minolta, Philips, Planmed and Siemens. For clinics having a variety of digital mammography systems, M-Vu can now provide a unified solution.
In addition to M-Vu CAD, VuCOMP has developed and commercialized a M-Vu Breast Density solution that received FDA market clearance in December 2013.
For more information: www.vucomp.com


 July 29, 2024
July 29, 2024