
Nuclear brain scan performed on a Biograph Vision positron emission tomography/computed tomography (PET-CT) system from Siemens Healthineers. It shows sharply defined cortical uptake with high contrast between gray and white matter.

Nuclear myocardial perfusion scan performed on a Biograph Vision positron emission tomography/computed tomography (PET-CT) system from Siemens Healthineers. The image shows good clarity with delineation of the left ventricular edge and papillary muscles without cardiac gating.
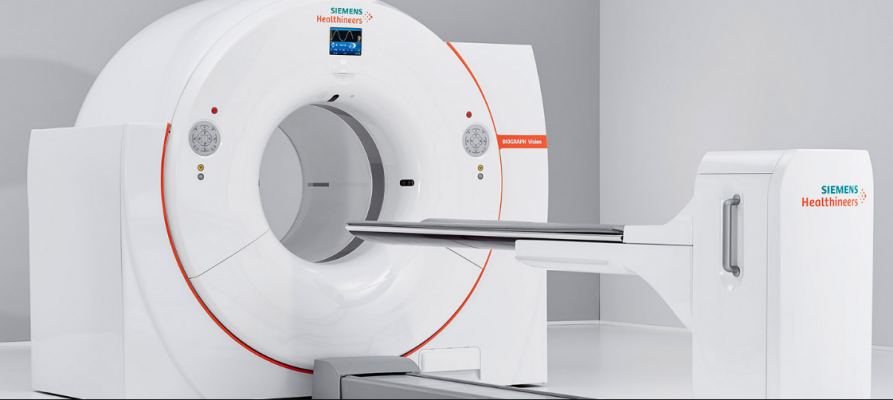
June 5, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Biograph Vision, a new positron emission tomography/computed tomography (PET-CT) system from Siemens Healthineers. The system offers new technologies to help improve image quality with motion management, improved delineation and quantification of small lesions, and new detector technology.
The Biograph Vision features new Optiso Ultra Dynamic Range (UDR) Detector Technology, which is based on silicon photomultipliers (SiPMs) rather than the photomultiplier tubes (PMTs) that have been the industry standard. This new system design enables Siemens to reduce the detector’s lutetium oxyorthosilicate (LSO) crystal elements from 4 x 4mm to 3.2 x 3.2mm, resulting in higher spatial resolution. Utilizing these extremely small LSO crystals and covering 100 percent of the area of the scintillator array with SiPMs, the Biograph Vision is designed to deliver industry’s fastest time-of-flight (ToF), with a temporal resolution of just 249 picoseconds, Siemens said. The system also provides a high effective sensitivity at 84 cps/kBq. For these reasons, the Biograph Vision helps to reduce scan time by a factor of 3.9 to improve patient throughput as well as reduce patient radiation exposure and tracer cost, Siemens said.
The Biograph Vision also has a large 78cm bore that offers 24 percent more space than a 70cm bore. This larger bore can reduce patient anxiety in addition to enabling easier positioning for radiotherapy (RT) devices or bariatric patients. The system fits into any room that houses a PET/CT scanner from the Biograph mCT family without the need for costly renovations.
Additionally, the Biograph Vision offers optional features to improve the quality of patient care. FlowMotion Multiparametric Suite, the industry’s first automated solution for whole-body parametric PET exams, provides not just the standard uptake volume (SUV), but also information regarding metabolic glucose rate and distribution volume. This information helps clinicians differentiate between non-metabolized and metabolized fluorodeoxyglucose (FDG) in the patient. OncoFreeze and CardioFreeze provide PET/CT images that are virtually free of motion in the same period of time as a regular whole-body scan, helping to improve lesion conspicuity, delineation, and quantification compared to images acquired without these features.
“The Biograph Vision represents a major leap in performance beyond any PET/CT system previously manufactured,” said Jim Williams, Ph.D., head of Siemens Healthineers molecular imaging. “With this system, we extend the boundaries of PET imaging and help our customers explore a new frontier in precision medicine.”
For more information: www.siemens-healthineers.com





 July 30, 2024
July 30, 2024