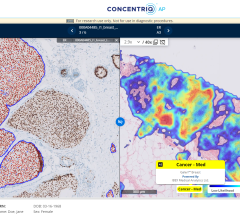
November 2, 2011 — InfiMed announced it has received U.S. Food and Drug Administration (FDA) 510(k) approval for its i5DR digital imaging software. The new program integrates with the Samsung SDX-4336CP portable flat panel detector.
The i5DR digital imaging system is a cost-effective solution with built-in flexibility for easy installation to any new or existing room, or mobile X-ray system.
For more information: www.infimed.com

 July 26, 2024
July 26, 2024