November 26, 2014 — The U.S. Food and Drug Administration (FDA) today cleared HeartFlow’s FFR-CT (fractional flow reserve—computed tomography) software, which permits non-invasive evaluation of coronary artery blood flow in patients showing signs of coronary artery disease.
The clearance may open a new era of coronary CT imaging, where a single scan can show blood flow with specific FFR flow rates for all segments in the heart. This may eliminate the need to perform diagnostic catheter angiography or to perform invasive catheter-based FFR tests. FFR-CT shows the exact area of blockages, the impact each has on flow and can help guide treatment and aid in interventional procedural navigation. Coronary CT experts predict the technology also may eliminate the need for myocardial perfusion nuclear imaging and other tests usually performed on patients presenting to emergency departments with chest pain.
Coronary artery disease is the leading cause of death for both men and women in the United States. One piece of clinical information healthcare professionals use to determine the extent of a blockage in the heart or a coronary artery is a value called fractional flow reserve (FFR). Obtaining this value requires an invasive cardiac catheterization.
The HeartFlow FFR-CT software can non-invasively provide an estimate of FFR using data from a CT scan of the patient’s heart. Doctors can use the estimate, along with other clinical patient data, to determine the likelihood that the actual FFR is below accepted limits and whether or not a more accurate FFR assessment using cardiac catheterization is necessary. It is expected the technology will greatly reduce the number of patients who are currently sent to the cath lab only to find their blood flow through a partial blockage is normal.
“Heart Flow FFR-CT is a computer modeling program that provides a functional assessment of blood flow in the coronary arteries from detailed anatomical data,” said William Missal, M.D., MPH, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health. “This non-invasive method is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures.”
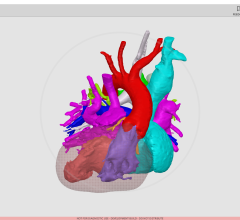
The Heart Flow FFR-CT software is housed at HeartFlow Inc.’s headquarters in Redwood City, Calif. A healthcare professional electronically sends the patient’s CT scan data to HeartFlow where a case analyst creates 3-D computer models of different sections of the patient’s heart and runs a blood flow simulator program on the models. After analyzing the data and the models, the case analyst electronically sends a report with the estimated FFR-CT values displayed as color images of the patient’s heart.
The FDA reviewed the data for HeartFlow FFR-CT through the de novo premarket review pathway, a regulatory pathway for some low- to moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.
Data submitted to support the safety and effectiveness of HeartFlow FFR-CT included clinical studies that compared FFR-CT measurements to FFR values directly measured by cardiac catheterization on subjects with suspected coronary artery disease who were therefore referred for catheterization and FFR. The results showed that Heart Flow FFR-CT was able to correctly identify 84 percent of the significant blockages identified by FFR as requiring intervention, and 86 percent of blockages identified by FFR as not requiring intervention. The company also submitted data and information showing how they have mitigated risks associated with the device, such as controlling for erroneous calculations that can lead to delayed or improper treatment.
Due to the potential impact the technology may have on cardiology, the FDA sent out its own press release on the technology prior to the company releasing any news.
For more information: heartflow.com









 August 09, 2024
August 09, 2024