October 11, 2012 — SuperSonic Imagine announced it has received U.S. Food and Drug Administration (FDA) clearance for the addition of a real-time, adjustable numerical scale (ANS) in meters per second for ShearWave Elastography (SWE) on the Aixplorer.
In 2009, SuperSonic Imagine launched its FDA-cleared Aixplorer ultrasound system, the only system on the market with an UltraFast platform, capable of acquiring images up to 20,000 Hz, 100 times faster than conventional ultrasound. With these capabilities, the Aixplorer can provide clinically detailed B-mode image quality and major technological achievements such as UltraFast Doppler and real-time SuperSonic SWE.
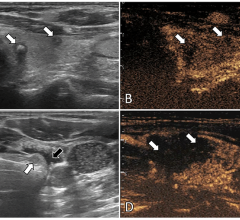
SuperSonic ShearWave Elastography uses shear wave velocity measurements to assess tissue stiffness in real time on a color-coded map. It has been used in various organs including the liver, prostate, thyroid and breast, with promising clinical results. As a user-skill independent, reproducible technique that is available on all of Aixplorer’s transducers, SWE has been a source of essential information for physicians as stiffer tissue can be related to various pathologies.
Now, with a real-time, adjustable numerical scale, Aixplorer can display shear wave velocity in meters per second using an optimal color-coded dynamic range. Clinicians can adjust the scale to optimize visualization of tissue elasticity according to the organ of interest for several clinical applications. For example, while imaging the liver, users will be able to easily and quickly adjust the scale when a liver nodule is seen in a fibrotic or cirrhotic context, as these nodules could be stiffer or softer than the surrounding tissue.
In addition, the real-time adjustable numerical scale will allow the user to repeat examinations with the same imaging display parameters. SuperSonic ShearWave Elastography is the first and only elastography the FDA has cleared with an adjustable numerical scale.
“This clearance is the result of a very constructive interaction with the FDA. We are also strongly involved in an effort launched by the RSNA [Radiological Society of North America] to promote the use of quantification of shear wave speed measurement as biomarkers in different pathologies” said Claude Cohen-Bacrie, executive vice president of SuperSonic Imagine.
Jacques Souquet, CEO of SuperSonic Imagine, added, “We are very excited that physicians in the [United States] will now have an additional tool to help them in their imaging of pathology. Our company strives to always bring the most innovative, clinically focused capabilities possible to ultrasound imaging.”
SuperSonic ShearWave Elastography imaging with a real-time adjustable numerical scale can be seen at RSNA 2012 in November.
For more information: www.supersonicimaging.fr


 July 19, 2024
July 19, 2024