November 24, 2014 — EDDA Technology announced that the company has received U.S. Food and Drug Administration (FDA) clearance on IQQA-BodyImaging. The new product will be introduced as the latest addition to the IQQA Platform and Product Suite for imaging-guided cancer treatment at the 100th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) in Chicago IL Nov. 30-Dec. 5. Also being introduced at RSNA is the company’s cloud-based OnDemand service with its clinical collaborator Massachusetts General Hospital.
The IQQA Platform and Product Suite was developed with a specific aim to maximize the utilization of multimodality imaging and its benefit for multiple stakeholders along the cycle of patient care, which include radiologists, interventional radiologists, surgeons and oncologists. By supporting efficient collaboration for optimal workflow and treatment management with clinical depth and OnDemand accessibility, the IQQA Platform enables a patient-specific, disease-targeted and multidisciplinary approach to cancer treatment.
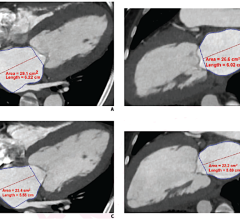
The IQQA-BodyImaging extends the 3-D features found in the IQQA product portfolio to thoracic, abdominal and pelvic multimodality scans. Features such as virtual knife for surgery and virtual needle for interventional procedural planning, monitoring and follow-up, provide comprehensive tools for tumor board assessment.
The company will also be demonstrating IQQA-Liver Multimodality, as part of the IQQA Product Suite. IQQA-Liver Multimodality is currently being used by physicians worldwide for real-time, interactive treatment planning and post-procedural assessment of liver diseases.
Through its proprietary IQQA Web-enabling technology and clinical partnership, EDDA offers OnDemand services for practices to use IQQA applications anytime and anywhere. “Through a combination of technology and in-depth clinical expertise, the MGH 3D Imaging Service offers access to our team of dedicated 3-D technologists to other hospitals and imaging centers with the goal of improving patient care by enhancing the efficiency, reliability, and scalability of 3-D imaging solutions for clinical decision support,” said Gordon J. Harris, Ph.D., director of the 3-D Imaging Service at Massachusetts General Hospital.
The IQQA Product Suite also includes IQQA-Guide (work-in-progress) for intraoperative 3-D navigation, IQQA-LiverFunction (work-in-progress) for localized hepatic functional analysis using 3-D SPECT, and IQQA-Chest CAD for small lung nodule detection. EDDA will exhibit its IQQA Platform and Product Suite in booth #4571 at RSNA 2014.
For more information: www.eddatech.com

 February 01, 2024
February 01, 2024