December 10, 2015 — EDDA Technology received FDA clearance on IQQA-Guide, a system that supports intra-operative precision 3-D navigation for surgical procedures of thoracic, abdominal and pelvic soft-organs.
IQQA-Guide is the latest addition to the IQQA platform and product suite. It expands the 3-D imaging analytics into the operating room. Fully quantified and patient-specific anatomic models are extracted from multi-modality images and utilized for on-the-fly 3-D navigation to support physicians in "seeing through" organ surfaces and quantitatively referencing anatomies of interest in 3-D during surgery. Clinical studies (WICO 2015) suggest that IQQA-Guide may help reduce the use of intra-operative CT scans during imaging-guided surgical procedures.
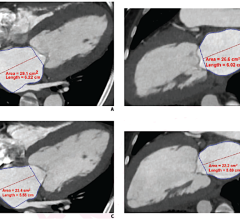
The treatment planning and follow-up assessment features provided by IQQA -BodyImaging, a part of the IQQA platform, have already been widely used for liver, kidney, and lung cancers. IQQA-BodyImaging has been proven to provide significant time-saving and improved patient care in more than 22,000 cases.
EDDA exhibited the new software at RSNA 2015.
For more information: www.eddatech.com

 February 01, 2024
February 01, 2024