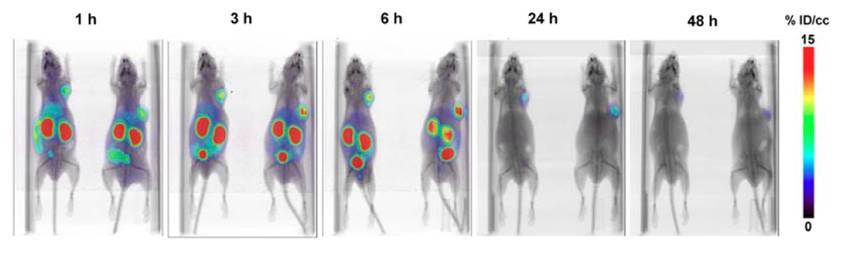
The mice were imaged with small-animal PET/CT using 124I-RPS-027 (7.4 MBq [200 μCi]). Credit: JM Kelly et al., Department of Radiology, Weill Cornell Medicine, New York, NY
September 14, 2017 — Researchers have demonstrated a new, effective way to precisely identify and localize prostate cancer tumors while protecting healthy tissue and reducing side effects. The new approach utilizes a single molecule designed to bind two proteins with differing affinities in an effort to optimize specific tumor localization — prostate-specific membrane antigen (PSMA) and human serum albumin — thereby enhancing tumor localization for targeted alpha radionuclide therapy. The study is presented in the featured basic article of the September issue of The Journal of Nuclear Medicine.
PSMA-targeted radiotherapy of prostate cancer is a promising approach to the treatment of disease that has spread. A small number of ligands have been evaluated in patients, and while early tumor response is encouraging, the relapse rate is high and these compounds can cause adverse side effects.
“We see now the pitfalls of PSMA targeting and have attempted to circumvent some of these by improved molecular design,” explained John W. Babich, Ph.D., professor of radiopharmaceutical sciences in radiology at Weill Cornell Medicine, New York. “We believe that our double targeting ligands are the first PSMA ligands designed to make use of the blood pool as a ‘safe zone’ reservoir to protect sensitive organs and tissues from off-target effects of alpha irradiation and, simultaneously, to reduce kidney localization while maintaining excellent tumor targeting.”
The goal is to develop a way of optimizing therapy by increasing the dose delivered to the tumor while simultaneously reducing damage to healthy tissue. To that end, the research team developed the double targeting ligand RPS-027, which binds to both PSMA and albumin. It was tested in mouse models of human prostate cancer, and the results, assessed via positron emission tomography/computed tomography (PET/CT), show high tumor uptake, with reduced kidney uptake; very favorable tissue distribution; and a promising therapeutic profile. In comparison to existing ligands proposed for targeted therapy of prostate cancer, RPS-027 has tumor-to-tissue ratios that predict a significant reduction in side effects during therapy. The researchers propose dual targeting ligands such as RPS-027 as next-generation radiopharmaceuticals for targeted alpha therapy using Astatine-211 (211At).
“Designing ligands that allow modulation of normal tissue localization can have a positive impact on the therapeutic index of alpha therapy and lead to widespread clinical utility,” Babich pointed out. “If our strategy can be applied successfully to other therapeutic targeting agents, we could expect an expansion in the use of targeted alpha therapy in the not-too-distant future.”
The use of theranostics (combined diagnostic targeting and therapy) for precision medicine is already revolutionizing the care of patients with thyroid cancer and neuroendocrine tumors. Studies such as this one show real promise for their use against prostate cancer and other diseases. They also highlight the key role of nuclear medicine in developing new therapies that improve patients’ lives.
For more information: www.jnm.snmjournals.org


 August 09, 2024
August 09, 2024