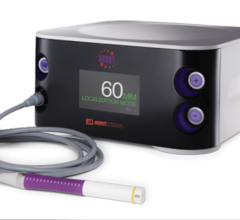
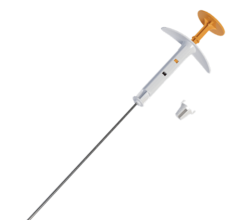
April 10, 2009 - Xoft Inc said that it has been granted a medical device license by the Medical Devices Bureau of Health Canada to sell and market the Axxent Electronic Brachytherapy System.
The proprietary Electronic Brachytherapy (eBx) treatment platform is designed to deliver localized, non-radioactive, isotope-free radiation treatment in minimally-shielded clinical settings under the supervision of a radiation oncologist to help reduce recurrence of cancer and improve survival.
Xoft also announced that it has received certification from the International Organization for Standardization (ISO) covering the design, development and manufacture of the Axxent Electronic Brachytherapy System and its components. Xoft has met the requirements of the ISO 13485:2003 standard.
For more information: www.xoftinc.com


 July 11, 2024
July 11, 2024