April 27, 2009 - An additional breast cancer was found in 9 percent of patients when breast-specific gamma imaging (BSGI) was used to complement mammography, according to a study from Beth Israel Medical Center, New York, presented at the American Society of Breast Surgeons 2009 Annual Meeting in San Diego.
The study found that of a total of 82 patients who underwent BSGI for newly diagnosed breast cancer, 18 had an additional abnormality, and 17 were biopsied.
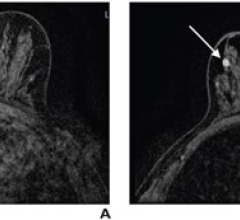
BSGI, a molecular breast imaging technique, is an adjunct to mammography that can see lesions independent of tissue density and discover early stage cancers. With BSGI, the patient receives a pharmaceutical tracing agent that is absorbed by all the cells in the body. Due to their increased rate of metabolic activity, cancerous cells in the breast absorb a greater amount of the tracing agent than normal, healthy cells and generally appear as "dark spots" on the BSGI image. The Dilon 6800 Gamma Camera is a high-resolution, compact gamma camera, optimized to perform BSGI.
"We wanted to determine the number of patients with known breast cancer who were found to have an additional lesion detected by BSGI, but undetected by mammography," said Susan K. Boolbol, M.D., chief of breast surgery at Beth Israel Medical Center, New York. "In our study group, 22 percent of patients had a change in surgical management based on BSGI findings. This is critical information in our desire to diagnose breast cancer early for successful treatment."
BSGI provides the capability of helping differentiate cancer from other structures or benign tissue in the breast. Unlike mammography, BSGI is not affected by tissue density. The test is especially useful for patients who have dense breasts, scar tissue, implants, or palpable lesions that cannot be detected using mammography or ultrasound.
Boolbol and her team conducted an IRB-approved review of all patients who underwent BSGI at Beth Israel Medical Center from 2005 to 2008. A total of 82 patients underwent BSGI for newly diagnosed breast cancer. There were five cases of invasive ductal carcinoma, two DCIS, one LCIS, two papillomas, and eight benign biopsies. One patient proceeded directly to mastectomy and an area of DCIS was found, which corresponded to the BSGI.
In the study group, 22 percent of patients had a change in surgical management based on BSGI findings. BSGI detected additional cancer in 9 percent of patients.
"We know that mammography will not detect all breast cancers. This study proves that BSGI is an additional tool to detect breast cancers otherwise missed by standard imaging. Therefore, BSGI plays an important role in the clinical management of patients with known breast cancer," said Dr. Boolbol.
For more information: www.dilon.com


 July 17, 2024
July 17, 2024