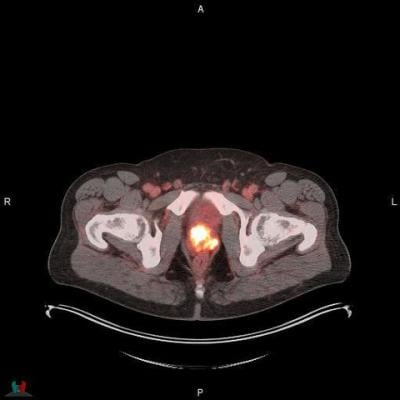
POSLUMA (flotufolastat F 18) PET/CT image showing uptake in the prostate gland, consistent with primary prostate cancer Photo courtesy of Blue Earth Diagnostics
July 28, 2023 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, announced that its optimized, high-affinity radiohybrid (rh) Prostate-Specific Membrane Antigen (PSMA)-targeted PET imaging agent, POSLUMA (flotufolastat F 18) injection (formerly referred to as 18F-rhPSMA-7.3), is now included in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer version 2.2023. POSLUMA is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. POSLUMA has been added to the NCCN guideline recommendations alongside and for all the same categories as other currently FDA-approved PSMA PET agents. The intent of the NCCN Guidelines is to assist in the decision-making process of individuals involved in cancer care—including physicians, nurses, pharmacists, payers, patients and their families—with the ultimate goal of improving patient care and outcomes.
POSLUMA was approved by the U.S. Food and Drug Administration (FDA) on May 25, 2023. It is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid (rh) technology. POSLUMA is widely available through 36 radiopharmacies in the national network of Blue Earth Diagnostics’ commercial U.S. manufacturer and distributor.
“The addition of POSLUMA to the highly respected NCCN Guidelines is a major milestone for Blue Earth Diagnostics,” said David E. Gauden, D. Phil., Chief Executive Officer of the Company. “We believe it further validates the clinical utility of POSLUMA in patients with newly diagnosed or recurrent prostate cancer, and can help expand patient access. In conjunction with our continuing efforts to increase U.S. commercial supply, Blue Earth is committed to make our new product widely available for patients and their physicians.”
“The NCCN Guidelines are widely used by clinicians and healthcare providers as a benchmark to assess clinical utility,” said Eugene J. Teoh, MBBS, MRCP, FRCR, D.Phil., Chief Medical Officer of Blue Earth Diagnostics. “POSLUMA was developed to assist physicians in the detection and localization of prostate cancer. This update recognizes the important ability of PSMA PET imaging procedures to detect and localize newly diagnosed and biochemically recurrent prostate cancer, which are essential to making appropriate patient management decisions. ”
The multidisciplinary NCCN panel, composed of experts from NCCN member organizations, based its review on the efficacy and safety data that formed the basis of the approval of POSLUMA by the U.S. FDA, as well as on the Phase 3 clinical trial results published recently in the Journal of Urology and European Urology.
The National Comprehensive Cancer Network (NCCN) is a not-for-profit alliance of leading cancer centers devoted to patient care, research, and education. NCCN is dedicated to improving and facilitating quality, effective, equitable, and accessible cancer care so all patients can live better lives.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) provide transparent, evidence-based, expert consensus recommendations for cancer treatment, prevention, and supportive services; they are the recognized standard for clinical direction and policy in cancer management and the most thorough and frequently-updated clinical practice guidelines available in any area of medicine.
About POSLUMA (flotufolastat F 18)
POSLUMA (flotufolastat F 18) injection (formerly referred to as 18F-rhPSMA-7.3) is an optimized, targeted radiohybrid diagnostic imaging agent indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. Precision PET imaging with POSLUMA can help identify the location and extent of prostate cancer, providing clinically valuable information to guide patient management. POSLUMA represents a new class of high-affinity PSMA-targeted PET radiopharmaceuticals based on novel radiohybrid technology and is labeled with the radioisotope 18F to provide readily available patient access and leverage the high image quality of 18F-labeled PSMA PET imaging to facilitate effective detection of disease. POSLUMA was approved by the U.S. Food and Drug Administration in May 2023.
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA) compounds consist of a radiohybrid (“rh”) Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells, and they may be radiolabeled with imaging isotopes for PET imaging, or with therapeutic isotopes for therapeutic use – providing the potential for creating a true theranostic technology. Radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA diagnostic imaging technology from Scintomics GmbH in 2018, and therapeutic rights in 2020, and sublicensed the therapeutic application to its sister company Blue Earth Therapeutics. Blue Earth Diagnostics received U.S. Food and Drug Administration approval for its radiohybrid PET diagnostic imaging product for use in prostate cancer in 2023. rhPSMA compounds for potential therapeutic use are investigational and have not received regulatory approval.
Indication and Important Safety Information About POSLUMA
INDICATION
POSLUMA (flotufolastat F 18) injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer
- with suspected metastasis who are candidates for initial definitive therapy
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
IMPORTANT SAFETY INFORMATION
- Image interpretation errors can occur with POSLUMA PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. The performance of POSLUMA for imaging metastatic pelvic lymph nodes in patients prior to initial definitive therapy seems to be affected by serum PSA levels and risk grouping. The performance of POSLUMA for imaging patients with biochemical evidence of recurrence of prostate cancer seems to be affected by serum PSA levels. Flotufolastat F 18 uptake is not specific for prostate cancer and may occur in other types of cancer, in non-malignant processes, and in normal tissues. Clinical correlation, which may include histopathological evaluation, is recommended.
- Risk of Image Misinterpretation in Patients with Suspected Prostate Cancer Recurrence: The interpretation of POSLUMA PET may differ depending on imaging readers, particularly in the prostate/prostate bed region. Because of the associated risk of false positive interpretation, consider multidisciplinary consultation and histopathological confirmation when clinical decision-making hinges on flotufolastat F 18 uptake only in the prostate/prostate bed region or only on uptake interpreted as borderline.
- POSLUMA use contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Advise patients to hydrate before and after administration and to void frequently after administration. Ensure safe handling to minimize radiation exposure to the patient and health care providers.
- The adverse reactions reported in ≥0.4% of patients in clinical studies were diarrhea, blood pressure increase and injection site pain.
- Drug Interactions: androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F 18 in prostate cancer. The effect of these therapies on performance of POSLUMA PET has not been established.
To report suspected adverse reactions to POSLUMA, call 1-844-POSLUMA (1-844-767-5862) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Full POSLUMA prescribing information is available at www.posluma.com/prescribing-information.pdf.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
For more information: www.blueearthdiagnostics.com
RELATED PROSTATE CANCER CONTENT:
Blue Earth Diagnostics Announces Key Results from Phase 3 SPOTLIGHT Study of 18F-rhPSMA-7.3


 December 04, 2025
December 04, 2025 









