Matakina International today announced a partnership with Microsoft Corporation to make its revolutionary breast density assessment software available to users globally via the Microsoft Azure Cloud. As a result of the partnership, the company launched a cloud-based version of its Volpara Breast Density Assessment software at the 98th annual meeting of the Radiology Society of North America, Nov. 25-30, 2012.
Cleared by the Federal Drug Administration (FDA), HealthCanada, the TGA and CE-marked, Volpara is in use at sites across the globe helping radiologists assess breast density more objectively and helping them better consider who might benefit from additional screening.
Until now, Volpara has been installed on individual workstations or as an enterprise-wide solution where the software is installed on a central server. Driven by use of Volpara in a number of large clinical trials and national breast cancer screening programs worldwide, the additional cloud-based option now offers secure, HIPAA-compliant access to the raw mammography acquisition data required to perform breast density assessment. And, for the first time, the cloud-based version also enables facilities and researchers to store unlimited data.
“The cloud also enables the ability to perform temporal analysis of breast density. Traditionally, facilities have not stored the raw data from which density measurement is performed. Now with long-term secure access to breast density data, an individual patient’s breast density values from one year could be compared with other years, which we believe could help detect cancers earlier by picking up the first signs of change,” said Prof. Martin Yaffe, professor of biomedical physics at the University of Toronto and one of the founders of Matakina.
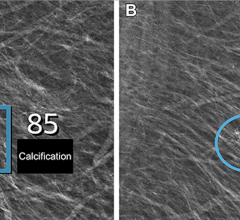
Volpara is a reliable tool which generates objective, automatic measurement of volumetric breast density values under the Volpara Density Grade (VDG) – a refined grading system which correlates with the American College of Radiology BIRADS Density Grading Classifications. Volpara is FDA cleared for all digital mammography units; integration with other digital mammography systems, computer-aided detection (CAD) systems and mammography reporting systems are also underway.
For more information: www.volparadensity.com


 July 29, 2024
July 29, 2024