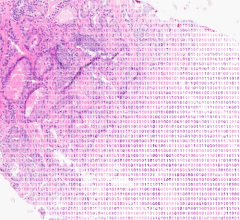
March 19, 2008 - Xoft Inc. received expanded clearance from the FDA for the Axxent Electronic Brachytherapy System, a proprietary technology platform designed to deliver localized, non-radioactive, isotope-free radiation treatment in minimally shielded clinical settings.
Previously cleared for accelerated treatment of early stage breast cancer, the Axxent System is now cleared for use in the treatment of other cancers or conditions where radiation therapy is indicated.
As a platform technology, the Axxent Electronic Brachytherapy System is designed to address oncological and non-oncological indications. Xoft is reportedly working to extend the use of Electronic Brachytherapy to endometrial and rectal indications, which are pending FDA clearance.
Designed to deliver electronic, X-ray-based radiation treatment, the Axxent treatment platform can be used in virtually any clinical setting under the supervision of a radiation oncologist, said the company. The Axxent System is designed to deliver non-radioactive therapy directly to cancer sites with minimal radiation exposure to surrounding healthy tissue. Reportedly eliminating the need for heavily shielded environments, it gives radiation oncologists the flexibility to deliver therapy in a broader range of clinical settings.
In its current use for the treatment of early stage breast cancer, the Axxent Electronic Brachytherapy System also provides the opportunity to reduce the therapy time required from seven weeks (for external radiation therapy) down to five days. This may accelerate patient choice of breast sparing lumpectomy surgery with adjuvant radiation therapy over the alternative of a full mastectomy.
For more information: www.xoftinc.com


 November 11, 2025
November 11, 2025