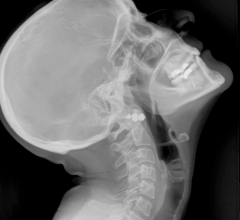
November 17, 2016 — Agfa HealthCare announced that it will introduce its new DR 800 X-ray room with Dynamic MUSICA at the 2016 annual meeting of the Radiological Society of North America (RSNA 2016). The multi-purpose solution covers radiography, fluoroscopy and advanced clinical applications.
The multi-purpose digital radiography suite is designed to enable radiology to meet the growing demand for fluoroscopy, without requiring multiple investments. The DR 800 is a robust and reliable solution that can handle a full range of radiographic exams (skeletal, thorax, abdomen, weight bearing) and fluoroscopy exams (including barium studies, arthrograms, cystograms, myelography and catheter placement, etc.).
The DR 800 comes with MUSICA high-speed, multi-scale image processing, which can now also process moving images. Along with enhanced noise suppression and superb brightness control, Dynamic MUSICA processing reduces veiling glare and plays a significant role in enabling potential dose reduction¹.
The DR 800 solution also offers high-quality radioscopy and fluoroscopy in supine and upright positions. With its 180 cm SID and auto-positioning presets, no compromises need to be made during chest imaging. The highly customizable DR 800 can be equipped with high-frequency fluoroscopy generator options of 50, 65 or 80 kW, a console for patient-side positioning, a wireless console for remote control, a compressor cone and an auto-switching anti-scatter grid. EasyStitch technology enables fast and high precision Full Leg Full Spine exams, while LiveVision technology allows for accurate dose-free remote positioning by providing a first-person camera view of the patient. Combined with MUSICA image processing, Advanced Cesium Iodide (CsI) detector technology offers high-speed, high-resolution imaging at low dose[1]. The flexible positioning, optimized workflow and premium image quality offered by Agfa's DR 800 solution, will enhance both efficiency and patient satisfaction.
The DR 800 X-ray room is still pending U.S. Food and Drug Administration (FDA) 510(k) submission and is not available in the United States and Canada.
For more information: www.agfahealthcare.com


 March 19, 2025
March 19, 2025