December 4, 2012 — GE Healthcare announced at RSNA 2012 receipt of U.S. Food and Drug Administration (FDA) clearance for its advanced imaging tool, FlightPlan for Liver. Developed to help make intricate liver embolization procedures simpler, FlightPlan for Liver has been commercially available in Europe, Latin America and Asia since 2011, with more than 30 installations in more than 10 countries.
Liver cancer is the fifth most common cancer in men (523 000 cases per year, 7.9 percent of the total) and the seventh in women (226 000 cases per year, 6.5 percent of the total)[1]. In response, liver embolization is a standard palliative treatment for liver cancer aiming at blocking the blood supply to the tumor. However, the identification of the vessels feeding the tumor can be difficult and time consuming due to the liver being highly vascularized in nature. Today, techniques using 2-D and 3-D imaging have limitations, often requiring significant amounts of time, radiation, and contrast media.
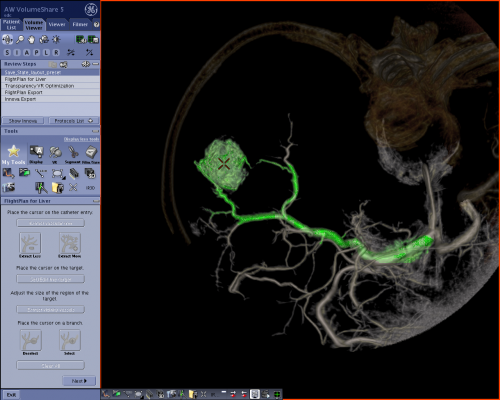
GE’s solution: Automatic identification of tumor vicinity vessels
GE Healthcare’s FlightPlan for Liver helps interventionalists plan their liver embolization procedures. The physician simply needs to select the tip of the catheter and a hypervascular tumor on a 3-D image, and let the software highlight the vessels traveling from the catheter to the lesion’s vicinity. The highlighted vessels can then be used as a 3-D roadmap with the Innova Vision application, and superimposed on the live fluoroscopic image to help the doctor guide the catheter into the target artery.
“The development of this technology for liver embolization is a great example of how collaborative work between physicians and engineers can help cancer patients. In three simple steps, the interventional radiologist can untangle the complex tumor vessels in the liver to immediately demonstrate which vessels feed the liver cancer and require catheter directed treatment,” said Stephen B. Solomon, chief of Interventional Radiology Service, Memorial Sloan-Kettering Cancer Center.
In effect, FlightPlan for Liver has the potential to extend the technique to more practitioners as it helps improve the confidence in performing this difficult procedure.
“This solution exemplifies our continuous efforts to provide to interventionalists solutions to plan, guide and assess their procedures. With FlightPlan for Liver, interventionalists can gain confidence in identifying tumor-feeding vessels and be more selective when planning liver embolization. With the collaboration of leading cancer institutions such as Memorial Sloan-Kettering Cancer Center (MSKCC), the Institut Gustave Roussy (IGR) and Beaujon Hospital, we can develop clinical tools that make a true difference to the doctors and more importantly to the patients,” said Chantal Le Chat, general manager, Premium Angiography, Detection and Guidance Solutions, GE Healthcare.
For more information: www.gehealthcare.com


 July 26, 2024
July 26, 2024 








