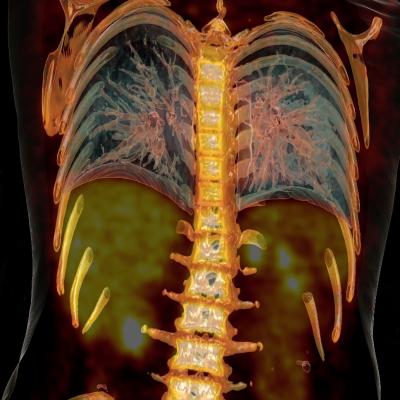
February 23, 2015 — New research from Johns Hopkins School of Medicine reveals a high value for scans which could lead to future change of reimbursement policies for follow-up positron emission tomography/computed tomography (PET/CT) studies in lung cancer. The study, featured in the February 2015 issue of The Journal of Nuclear Medicine, establishes the value of fourth and subsequent follow-up PET/CT scans in clinical assessment and management change in patients with the disease.
In the retrospective study, a total of 1,171 patients with biopsy-proven lung cancer who had positron emission tomography with a radioactive tracer (18F-FDG) were identified at a single tertiary center from 2001 to 2013. Among these, 85 patients (7.3 percent) had four or more follow-up PET/CT scans with a total of 285 fourth and subsequent follow-up PET/CT scans. Median follow-up from the fourth scan was 31.4 months. The follow-up PET/CT scan results were correlated with clinical assessment and treatment changes.
Of the 285 fourth and subsequent follow-up PET/CT scans, 149 (52.28 percent) were interpreted as positive and 136 (47.7 percent) were interpreted as negative for recurrence or metastasis. A total of 47 (55.3 percent) patients died during the study period. PET/CT identified recurrence or metastasis in 44.3 percent of scans performed without prior clinical suspicion and ruled out recurrence or metastasis in 24.2 percent of scans performed without prior clinical suspicion. The PET/CT scan resulted in treatment change in 28.1 percent (80/285) of the patients. New treatment was initiated in 20.4 percent (58/285) scan times, treatment was changed in 5.6 percent (16/285) scan times and ongoing treatment was stopped in 2.1 percent (6/285) scan times.
“Although the value of FDG PET/CT has been established in the follow-up of patients with lung cancer, this study specifically looks at the value of the fourth and subsequent follow-up PET/CT studies, which has not been addressed in any prior studies,” stated Rathan Subramaniam, M.D., Ph.D., lead author of the study. “We now need to establish similar evidence for other human solid tumors,” Subramaniam continued.
The study was performed in the context of the recent Center for Medicaid and Medicare Services declaration that only three FDG PET/CT scans will be covered under section 1862(a)(1)(A) when used to guide subsequent management of anti-tumor treatment strategy after completion of initial anticancer therapy. Coverage of any additional FDG PET/CT scans will be determined by local Medicare administrative contractors.
For more information: www.snmmi.org


 September 12, 2024
September 12, 2024 








