October 26, 2020 — Adding the advanced PET radiotracer fluciclovine to conventional imaging to help guide radiation treatments for recurrent prostate cancer can improve disease-free survival rates, a new study finds. Among patients whose prostate cancer had returned after surgical removal of their prostate, 75.5% whose treatment integrated the PET molecular imaging were disease-free after three years, compared to 63% for whom only conventional imaging techniques were used to plan treatment. The increased survival rate persisted for up to four years. Findings from the randomized phase II/III EMPIRE-1 trial (NCT01666808) was presented today at the American Society for Radiation Oncology (ASTRO) Annual Meeting (ASTRO).
“The decision to offer post-prostatectomy radiation therapy is complex, because conventional imaging can leave unanswered questions on the best approach to treatment planning,” said co-principal investigator Ashesh B. Jani, M.D., FASTRO, a professor of radiation oncology at the Winship Cancer Institute of Emory University in Atlanta. “What this research has found is that integrating advanced molecular imaging into the treatment planning process allows us to do a better job of selecting patients for radiation therapy, guiding radiation treatment decisions and planning and ultimately, keeping patients' cancer under control."
“This is the first study of its kind to look at the role of PET in influencing a cancer control endpoint,” added David M. Schuster, M.D., a nuclear radiology specialist and professor at Emory University who collaborated with Jani on the study as co-principal investigator. “That’s a very high bar for an imaging study.”
Prostate cancer is one of the most common types of cancer, and a leading cause of cancer death, among men in the United States. An estimated one in nine men will be diagnosed with prostate cancer in his lifetime. Removing all or part of the prostate is a common treatment, but for 20-40% of men, prostate specific antigen (PSA) levels may nonetheless rise after surgery, signaling a return of cancer cells. Furthermore, failure rates for post-surgical radiation treatments remain high. "This is prostate cancer that, by virtue of being recurrent, has demonstrated its capacity to be aggressive," explained Dr. Jani.
Conventional imaging techniques, such as bone scans, computed tomography (CT) or magnetic resonance imaging (MRI), are typically used to guide radiation therapy decisions and treatment plans for these recurrent cancers by identifying if tumors are present in the body. Molecular imaging with positron emission tomography (PET) scans, which look instead for higher levels of injected radiotracer activity within cancer cells, has been effectively used to guide treatment of many cancers by more accurately detecting the presence and location of developing tumors. Though previous PET studies have led to changes in how prostate cancer is managed, an ensuing improvement in patient outcomes had not been demonstrated until this point.
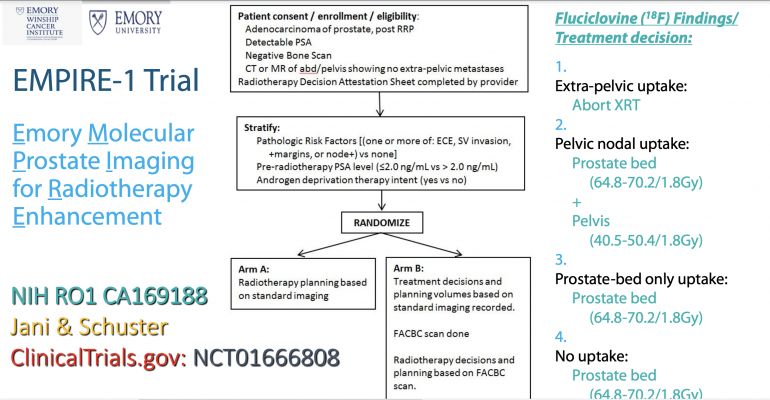
This study used a type of PET known as [fluorine-18, or 18F] fluciclovine (brand name Axumin). For this scan, patients are given radioactive tracers containing a synthetic amino acid. Because cancer cells use more amino acids than other cells, the cancer is more easily detected when it accumulates more of this type of radiotracer.
“This is a jump ahead of the traditional way of looking at things,” said Jani. “It helps us to pinpoint the location of the prostate cancer recurrence.” Research by the team previously established a role for fluciclovine in guiding post-prostatectomy radiation therapy treatment decisions and target volumes.
The Emory Molecular Prostate Imaging for Radiotherapy Enhancement, or EMPIRE-1 trial (NCT01666808), enrolled 165 patients who had undergone prostatectomies, but who later showed abnormal PSA blood test scores, indicating their cancer was returning. All patients underwent conventional imaging (bone scans, CT or MRI) for initial treatment planning. Patients were then randomized into two groups. The first group received radiation therapy based on these initial treatment plans. The other group was given fluciclovine PET scans and treated based on the additional findings.
After three years, the study showed patients who were treated based on the more advanced imaging results had a higher disease-free survival rate (p=0.003), which persisted for four years (51% for those in the conventional arm, versus 75.5% in the advanced imaging arm, p<0.001). There were no significant differences in provider-reported severe genitourinary or gastrointestinal side effects between the two arms.
Researchers attributed the improved outcomes to better patient selection, by excluding patients with extra-pelvic disease who are more susceptible to failure, as well as better definition of the tumor and treatment field.
“The reason we did this research was because we were frustrated that we could not get better cure rates for patients with this type of cancer,” said Schuster. “We thought one of the factors involved may be the sensitivity of the imaging tests used for planning radiation therapy. We thought this new PET test, with its greater sensitivity for detecting prostate cancer, would translate into a better cure rate, as well. We believed there would be some effect but were pleasantly surprised by the strength of the findings.”
Fluciclovine was approved for use by the U.S. Food and Drug Administration (FDA) about halfway through the study in 2016, and it is now widely accepted for the restaging of recurrent prostate cancer with inclusion in the NCCN guidelines. It has not yet been widely adopted as the standard for planning treatment in this patient population, however.
"Before a patient is considered for radiation therapy to treat prostate cancer that has returned after surgery, the doctor should not rely on traditional imaging alone, but should also use advanced molecular imaging. Doing so will help determine if radiation therapy is appropriate for that patient and, if so, more precisely plan how to target his disease,” said Jani.
The collaborative team led by Drs. Jani and Schuster is already investigating whether a newer type of PET scan, which uses a radiotracer to target a receptor on the surface of prostate cancer cells, could be even more effective than fluciclovine in improving cancer control. The newer scan, PSMA, is not yet FDA approved.
“Fluciclovine PET has become the best available test,” said Schuster. “But now there’s an even newer kid on the block. In EMPIRE-2, we’re building on the results of EMPIRE-1 by comparing PSMA with fluciclovine to see which one of these radiotracers improves cancer control better.”
For more information: www.astro.org


 December 04, 2025
December 04, 2025 









