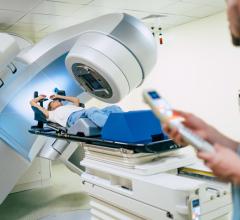
Image courtesy of Accuray Inc.
June 23, 2015 - Accuray Inc. announced in April that the InCise Multileaf Collimator (MLC) for the CyberKnife M6 System is available outside the United States in markets where it is approved for sale. The InCise MLC was launched in the U.S. market in February.
The launch follows the successful completion of the evaluation of the InCise MLC by Erasmus MC Cancer Institute in Rotterdam, the Netherlands, the European evaluation site working in collaboration with Accuray. Two sites in the U.S. had previously completed their successful evaluation of the InCise MLC. The main benefits of the MLC reported by the sites were the ability to treat a broader range of tumors than they could with fixed collimators or the Iris Collimator, and to do so with significantly increased efficiency. The InCise MLC was featured at the European Society for Therapeutic Radiology and Oncology (ESTRO) meeting in Barcelona, Spain, April 24 - 27.
The InCise MLC is one of the world's first multileaf collimators to be available on a robotic platform, the CyberKnife M6 System. The system's robotic design enables physicians to find the best angles for radiation to enter and exit the body, thus maximizing dose to the target while minimizing exposure to healthy tissue. The robotic design also enables the system to adjust and automatically stay on target in real-time, accounting for patient and tumor motion.
The CyberKnife M6 System can be used to treat tumors anywhere in the body, including the prostate, lung, brain, spine, liver, pancreas and kidney, and may offer hope to patients who have inoperable or surgically complex tumors, or who may prefer a clinically effective, non-surgical option.
For more information: www.accuray.com


 August 09, 2024
August 09, 2024