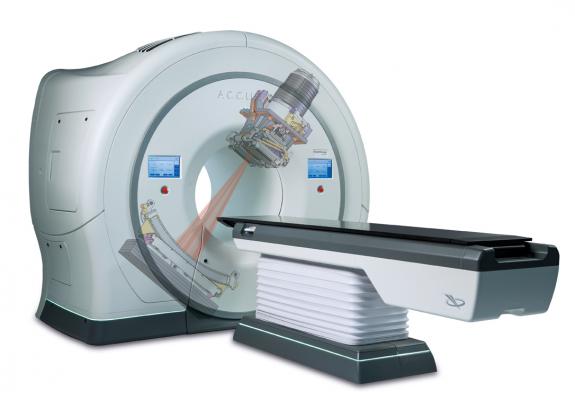
May 29, 2014 — Accuray Inc. announced the installation of its 500th TomoTherapy system at the grand opening of Palo Verde Cancer Center – Scottsdale in Scottsdale, Ariz. The system, a TomoHDA model, is the only radiation therapy device at the new center, which also offers medical oncology services. The center’s decision to invest in the latest TomoTherapy offering reflects growing confidence industry-wide in the enhanced performance and clinical versatility of the technology, long known for its superior radiation therapy delivery, precision and treatment quality.
The TomoHDA System offers fast, reliable, accurate and flexible treatment planning and delivery for each patient, regardless of the location, size and complexity of the tumor. It is an ideal option for treating a wide range of cancers, including recurrent disease in patients who have already received maximum radiation doses to critical organs.
“We analyzed all radiation therapy offerings when planning our new, fully-integrated cancer center, and chose the TomoHDA system for its outstanding speed, performance and simplicity,” said Lauren Stegman, medical director of radiation oncology at Palo Verde Cancer Center – Scottsdale. “We wanted technology that would allow us to treat the broadest range of patients, and we find TomoHDA treatment planning to be flexible and easy-to-use. After working with older TomoTherapy equipment for nearly a decade and with the Accuray CyberKnife System at our affiliate site, the Phoenix CyberKnife and Radiation Oncology Center, we are confident that this next generation TomoHDA technology will allow us to treat more patients effectively. The System is the perfect complement to our existing treatment offerings and will further ensure we won’t have to turn away any difficult-to-treat patients.”
For more information: www.accuray.com


 April 21, 2025
April 21, 2025 








