March 4, 2011 – A 3-D mammography system and other ready-to-market technologies in women’s imaging will be showcased at the National Interdisciplinary Breast Center Conference (NCBC) in Las Vegas.
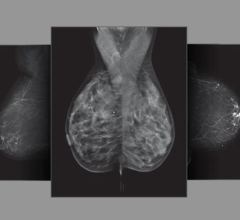
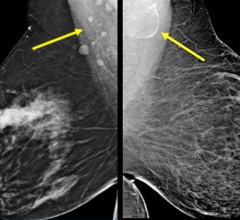
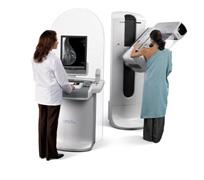
Hologic's Selenia Dimensions technology is the first commercially available breast cancer screening and diagnosis system to deliver breast tomosynthesis. Mammography systems using conventional 2-D imaging have limitations caused by tissue overlapping tissue in the breast that may hide lesions or cause benign areas to appear suspicious.
In clinical studies, radiologists reading 2-D plus 3-D mammography (breast tomosynthesis) compared to 2-D mammography alone demonstrated superior clinical performance in specificity. They also had the confidence to rule out breast cancer without recalling the patient for further study, and demonstrated improved sensitivity, the proportion of mammograms which include breast cancers that were correctly diagnosed.
The system was approved for sale in the United States by the U.S. Food and Drug Administration (FDA) in February 2011.
It also gives radiologists the option of offering their patients a conventional 2-D digital mammogram and a 3-D tomosynthesis exam -- all under one compression. It takes just seconds, at a combined X-ray dose below the FDA's acceptable maximum guidelines for screening mammography.
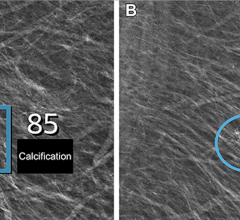
Also shown for the first time at NCBC are two new products designed specifically for the Selenia Dimensions 2D/3D system: the Affirm upright breast biopsy guidance system and ImageChecker 3D Calc computer-aided detection. Affirm is cleared for sale in the United States. ImageChecker 3D Calc is a work in progress and is not available for sale in the United States.
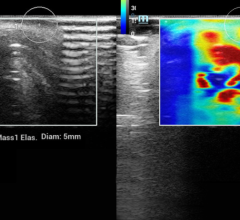
With the recent acquisition of Sentinelle Medical, Hologic is also highlighting a suite of breast magnetic resonance imaging (MRI) tools. MRI is an important standard of care in breast screening for women indicated as having a high risk of developing breast cancer.
For more information: www.hologic.com


 July 29, 2024
July 29, 2024