If you enjoy this content, please share it with a colleague
RELATED CONTENT
August 11, 2020 — SyntheticMR announced its imaging software SyMRI is compatible with additional scanners from Siemens ...
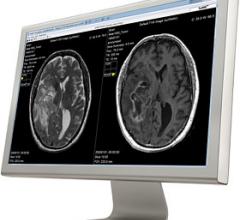
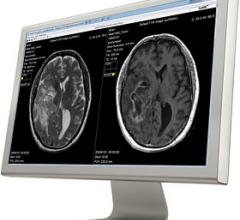
SyntheticMR announced U.S. Food and Drug Administration (FDA) clearance for clinical use of its SyMRI Image and SyMRI Neuro product packages together with magnetic resonance imaging (MRI) systems from Siemens Healthineers.
April 19, 2017 — REMyDI (Rapid Estimation of Myelin for Diagnostic Imaging), first introduced by SyntheticMR AB at the ...
SyntheticMR AB introduced REMyDI for automatic quantification of myelin volume in the brain at the 2016 annual meeting of the Radiological Society of North America (RSNA) in November. Easy quantification of myelin allows clinicians to follow myelination in the developing brain and monitor myelin degeneration in patients with demyelinating and neurodegenerative disorders. REMyDI is a feature of the SyMRI post-processing software from SyntheticMR.
SyntheticMR and The University of Texas MD Anderson Cancer Center have signed a research agreement to study SyMRI to characterize brain tumors.
Linköping University Hospital in Sweden has installed SyMRI Neuro from SyntheticMR in order to improve the follow-up of patients with multiple sclerosis (MS).
Cincinnati Children’s Hospital Medical Center (CCHMC) has evaluated synthetic magnetic resonance imaging (MRI) on pediatric cases to study and clinically validate its use on children. Results from the first part of the evaluation, which used SyMRI software, shows the synthetic images are diagnostically satisfactory in comparison with conventional sequences.


 August 11, 2020
August 11, 2020