If you enjoy this content, please share it with a colleague
ScreenPoint Medical
RELATED CONTENT
Feb. 5, 2025 — Results of a new randomized trial involving more than 105,000 women reveals that the use of artificial ...
Jan. 8, 2025 — ScreenPoint Medical has acquiredf Biomediq A/S, a research-based company focused on the research ...
Nov. 19, 2024 — Results of a UCLA study recently published in the Journal of Breast Imaging demonstrates that Transpara ...
March 13, 2024 — ScreenPoint Medical has announced that its industry leading Transpara Breast AI is available to improve ...
May 25, 2023 — Researchers from the Mayo Clinic (Rochester, MN) and University of California, San Francisco confirmed ...
September 2, 2020 — Diagnostic Centers of America (DCA) and Boca Radiology Group have partnered with ScreenPoint Medical ...
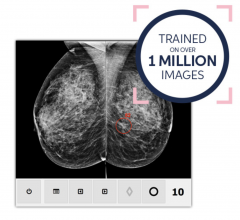
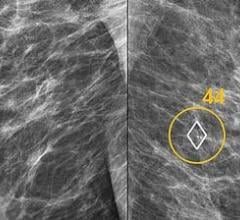
Volpara Solutions and ScreenPoint Medical BV signed an agreement under which Volpara will sell ScreenPoint's Transpara products to breast imaging clinics in the United States, Australia, New Zealand and parts of Asia. Transpara is designed to assist radiologists with the reading of mammograms and is one of the first next-generation artificial intelligence (AI) applications for detecting breast cancer in screening mammograms to gain 510(k) clearance from the U.S. Food & Drug Administration (FDA).
Siemens Healthineers showcased the new planned artificial intelligence (AI)-based features with its mammography reading and reporting solution, syngo.Breast Care, at the 2018 Radiological Society of North America (RSNA) annual meeting, Nov. 25-30 in Chicago.1 These features are designed to provide physicians with interactive decision support.
ScreenPoint Medical has signed a memorandum of understanding (MOU) with Volpara Health Technologies. Volpara will deliver ScreenPoint’s artificial intelligence (AI)-based products to customers as part of its expanding VolparaEnterprise AI cloud ecosystem. Together, the two companies expect to improve the clinical performance of mammography and drive the earlier detection of breast cancer. The partnership will leverage Volpara’s growing footprint in the breast imaging marketplace and will provide an opportunity for ScreenPoint to enter the U.S. market.
November 28, 2018 — ScreenPoint Medical announced it has received 510(k) clearance from the U.S. Food & Drug ...


 February 05, 2025
February 05, 2025