If you enjoy this content, please share it with a colleague
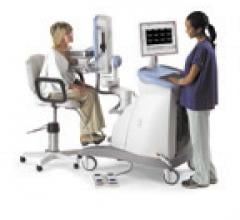
Naviscan PET Systems
RELATED CONTENT
March 15, 2010 - Researchers released new data from an National Institute of Health (NIH) sponsored, multi-site study of hundreds of women with newly diagnosed breast cancer shows that positron emission mammography (PEM) may reduce unnecessary breast biopsies.
January 20, 2010 - First Coast Oncology in Jacksonville, Fla., employs a technique for whole breast irradiation that is designed to target and deliver a boost dose accurately and reliably to the lumpectomy cavity margin.
December 22, 2009 – The Baylor University Medical Center in Dallas has taken delivery of the Naviscan PEM (positron emission mammography) scanner at its Darlene G. Cass Women’s Imaging Center.
The Naviscan PEM scanner is an optimized PET scanner designed to provide unprecedented metabolic visualization of small body parts. Through a unique combination of gentle immobilization, advanced photonics and image processing, the scanner provides 3-D tomographic images with resolution down to 2 mm.
June 24, 2009 - Naviscan Inc., a molecular breast imaging (MBI) provider, has entered into a distribution agreement with Hae Dong Co. Ltd. to market and serve Naviscan’s high-resolution organ-specific PET scanner, whose breast application is known as positron emission mammography (PEM), in the Republic of South Korea.
June 12, 2009 – Naviscan Inc. announced that it has issued a non-exclusive licensing agreement with an undisclosed imaging company for intellectual property enabling Naviscan's Stereo Navigator PEM-guided biopsy accessory, and will be demonstrating the technology at the SNM's 56th Annual Meeting Annual Meeting, Toronto, June 13 - 16, 2009, in booth 638.
June 2, 2009 - Naviscan Inc. has issued a nonexclusive licensing agreement with an undisclosed imaging company for intellectual property that enables Naviscan’s Stereo Navigator PEM-guided biopsy accessory to obtain emission data, locate the abnormal tissue and process the data to guide a biopsy needle.
Mammography is the one breast cancer screening test proven to reduce mortality, yet on many occasions, it still does not capture the full picture. Detection using the high sensitivity of breast-MRI (magnetic resonance imaging) is very promising, yet it often casts too wide a net.
November 20, 2008 - Naviscan Inc. said the FDA has granted 510(k) clearance for its biopsy-guidance feature designed exclusively for use with its high-resolution organ-specific PET scanner, Positron Emission Mammography (PEM).
Naviscan Inc. said the FDA has granted 510(k) clearance for its biopsy-guidance feature designed exclusively for use ...


 March 15, 2010
March 15, 2010