If you enjoy this content, please share it with a colleague
RELATED CONTENT
September 13, 2021 — Lantheus Holdings, Inc. and RefleXion Medical, Inc., today announced a development and ...
Here are several updates in medical imaging contrast media agents. Two of the biggest news items were related to safety ...
Lantheus Medical Imaging Inc. announced U.S. Food and Drug Administration (FDA) approval of a label update for Definity Vial for (Perflutren Lipid Microsphere) Injectable Suspension. The update removes the contraindication statement related to use in patients with a known or suspected cardiac shunt from the U.S. Prescribing Information.
Lantheus Holdings Inc. announced in late September that sub-analysis data from the first Phase 3 study of flurpiridaz F-18 for myocardial perfusion imaging (MPI) in patients undergoing exercise stress testing. The data was presented at the 21st annual scientific session of the American Society of Nuclear Cardiology (ASNC), Sept. 22-25 in Boca Raton, Fla.
Lantheus Holdings Inc., the parent company of Lantheus Medical Imaging Inc. (LMI), announced the first commercial shipment of Xenon Xe 133 gas (Xenon 133) using unprocessed radiochemical Xenon 133 supplied by the Institute for Radioelements (IRE) in Belgium.
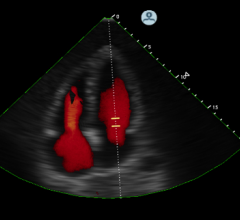
Breakthrough Research Demonstrates Contrast Ultrasound Can be Used as Heart Attack Treatment
More than 50 companies and organizations will display their latest products and services at the American Society of Echocardiography’s (ASE) 26th Annual Scientific Sessions, June 12-16 at in Boston, Mass. ASE 2015 is the world’s premier meeting for cardiovascular ultrasound practitioners, and promises a wealth of cutting-edge education, research, and the latest vendor technology.
Lantheus Medical Imaging, Inc., a developer, manufacturer and distributor of diagnostic imaging agents, announced preliminary results from the first of two planned Phase 3 trials to assess the diagnostic efficacy of flurpiridaz F 18, an imaging agent used in positron emission tomography (PET) myocardial perfusion imaging (MPI) for the detection of coronary artery disease (CAD).
Myocardial perfusion imaging (MPI) with positron emission tomography (PET) has been shown to be superior to single photon emission computed tomography (SPECT). Nevertheless, widespread clinical use of PET MPI has been limited by the currently available PET myocardial perfusion tracers.
Just when positron emission tomography (PET) appears to be eclipsing single photon emission computed tomography (SPECT) for cardiac imaging, new advances make SPECT more attractive. Both modalities also have suffered setbacks with radiopharmaceutical supply problems in recent years and both modalities have their pros and cons. Looking toward the future, the question of which modality will dominate remains unanswered. PET shows major promise with exciting new tracers, while new SPECT scanner technology introduced at the Society of of Nuclear Medicine and Molecular Imaging (SNMMI) 2013 meeting may herald a rebirth for SPECT with previously unseen image quality enhancements.

 September 13, 2021
September 13, 2021